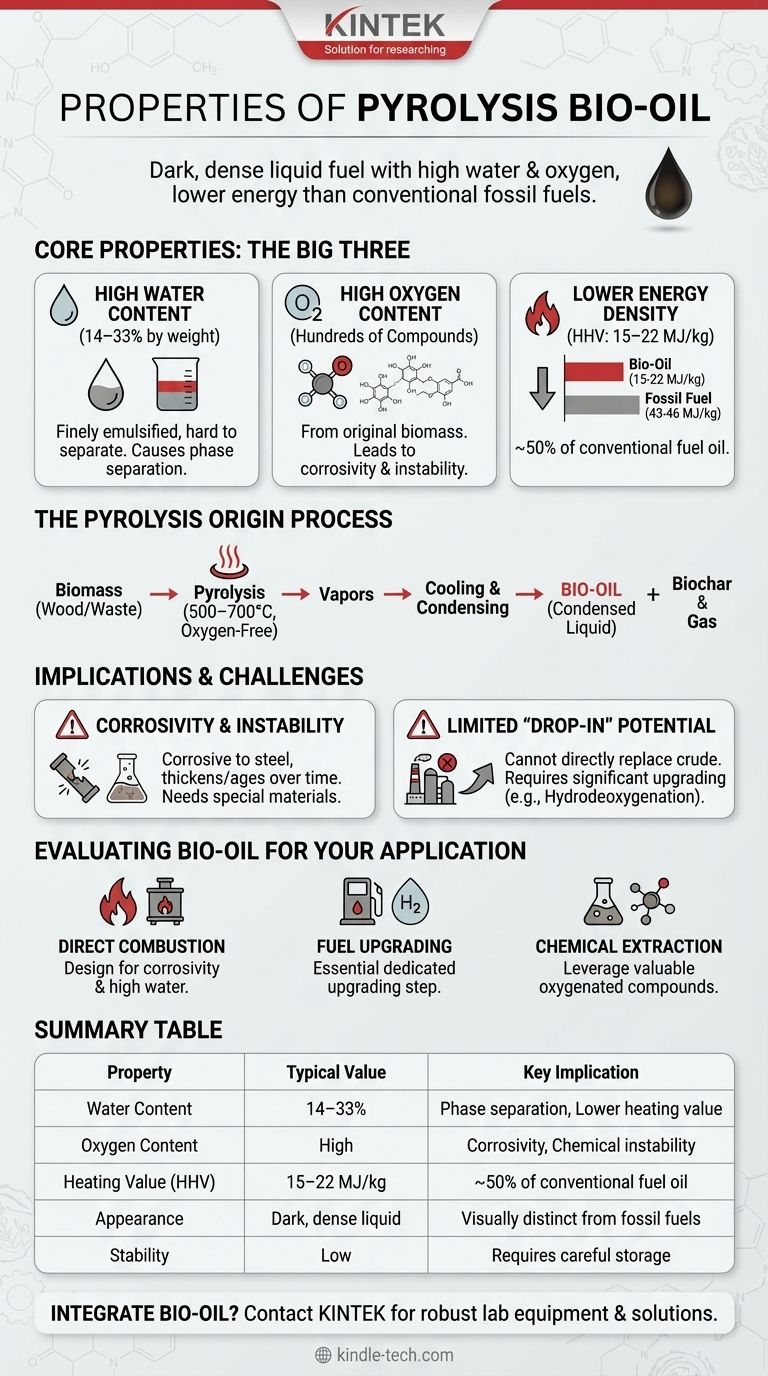
In essence, pyrolysis bio-oil is a dark, dense liquid fuel characterized by high water content, high oxygen content, and a lower energy value compared to conventional fossil fuels. This unique chemical profile stems directly from its origin as plant or organic matter and the rapid, oxygen-free heating process used to create it. While it is a renewable liquid, it is not a direct "drop-in" replacement for crude oil and has very distinct properties.
The defining characteristic of pyrolysis bio-oil is its high concentration of oxygenated compounds. This single factor is responsible for its lower heating value, chemical instability, and the primary challenges associated with upgrading it into conventional transportation fuels.

The Origin: How Pyrolysis Creates Bio-Oil
To understand bio-oil's properties, we must first look at how it's made. The process itself dictates the final chemical makeup of the liquid.
The Pyrolysis Process
Pyrolysis involves heating biomass, such as wood or agricultural waste, to high temperatures (500°C–700°C) very quickly in an environment without oxygen.
This intense, oxygen-free heat breaks down the complex organic structures of the biomass.
The Resulting Products
The process creates three primary products: pyrolysis vapors, non-condensable gases, and a solid carbon-rich material called biochar.
The hot vapors are then rapidly cooled and condensed. This condensed liquid is what we call pyrolysis bio-oil, or sometimes bio-crude.
Key Chemical and Physical Properties
Bio-oil's properties differ significantly from petroleum crude oil. These differences are critical for any practical application, from combustion to chemical production.
High Water Content
Bio-oil contains a significant amount of water, typically 14–33% by weight. This water is not easily separated by simple distillation because it is finely emulsified and chemically bound within the complex mixture.
At higher concentrations, this water can cause the oil to separate into distinct phases, creating storage and processing challenges.
High Oxygen Content
Unlike conventional fuels, which are almost entirely hydrocarbons, bio-oil contains a high percentage of oxygen. This is because the original biomass is rich in oxygen.
The oxygen is present in hundreds of different chemical compounds, such as acids, alcohols, aldehydes, and ketones. This high oxygen content is the most fundamental difference between bio-oil and fossil fuels.
Lower Energy Density
The high oxygen and water content directly results in a lower energy value. The Higher Heating Value (HHV) of bio-oil is 15–22 MJ/kg.
This is roughly half the energy density of conventional fuel oil, which has an HHV of 43–46 MJ/kg. In practical terms, you would need nearly twice the volume of bio-oil to produce the same amount of heat.
Understanding the Practical Implications
These properties create specific challenges and trade-offs that must be managed when using bio-oil. It is not a simple substitute for diesel or heating oil.
Corrosivity and Instability
The presence of organic acids and other reactive oxygenated compounds makes raw bio-oil corrosive to common construction materials like carbon steel.
Furthermore, these compounds can continue to react with each other during storage, causing the oil to thicken, polymerize, and age over time, altering its properties.
Limited "Drop-In" Potential
Because of its high oxygen content, corrosivity, and instability, bio-oil cannot be processed in a traditional petroleum refinery without significant pre-treatment.
Attempting to do so would damage catalytic equipment and yield poor results. It must first be "upgraded" in a process that removes oxygen, typically using hydrogen.
Evaluating Bio-Oil for Your Application
Your intended use for bio-oil will determine which of its properties are most important and which challenges must be overcome.
- If your primary focus is direct combustion for heat: Acknowledge its lower heating value means you will need a larger volume of fuel, and the system must be designed to handle its corrosivity and high water content.
- If your primary focus is upgrading to transportation fuels: Recognize that a dedicated upgrading step, like hydrodeoxygenation, is essential to remove oxygen and stabilize the oil before it can be co-processed in a refinery.
- If your primary focus is extracting specialty chemicals: Leverage the oil's unique composition by developing processes to isolate valuable oxygenated compounds, turning a fuel challenge into a chemical opportunity.
Understanding these core properties is the critical first step toward harnessing the potential of bio-oil as a sustainable resource.
Summary Table:
| Property | Typical Value / Characteristic | Key Implication |
|---|---|---|
| Water Content | 14–33% by weight | Phase separation, lower heating value |
| Oxygen Content | High (from biomass) | Corrosivity, chemical instability |
| Heating Value (HHV) | 15–22 MJ/kg | ~50% of conventional fuel oil |
| Appearance | Dark, dense liquid | Visually distinct from fossil fuels |
| Stability | Low (ages over time) | Requires careful storage and handling |
Ready to integrate pyrolysis bio-oil into your research or production process? KINTEK specializes in providing the robust lab equipment and consumables needed to safely handle, analyze, and upgrade bio-oil. Whether you're focused on combustion, fuel upgrading, or chemical extraction, our solutions help you navigate its unique properties effectively. Contact our experts today to discuss how we can support your sustainable energy projects with reliable laboratory technology.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Custom PTFE Teflon Parts Manufacturer for Culture Dish and Evaporation Dish
- Customizable CO2 Reduction Flow Cell for NRR ORR and CO2RR Research
People Also Ask
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas











