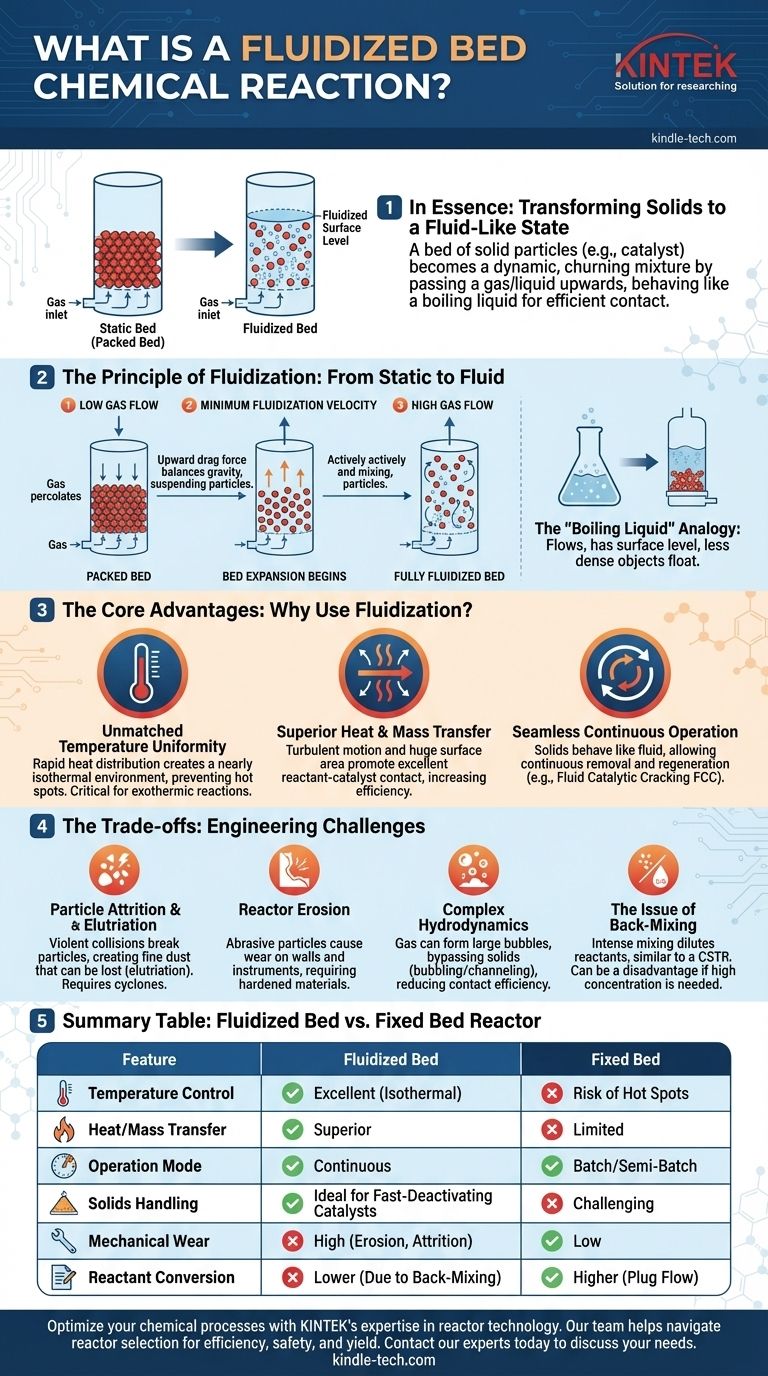
In essence, a fluidized bed reaction is a process where a bed of solid particles, such as a catalyst, is transformed into a fluid-like state by passing a gas or liquid up through it. This suspension of solids within the fluid creates a dynamic, churning mixture that behaves like a boiling liquid, allowing for incredibly efficient contact between the reactants and the solid catalyst.
Fluidized bed reactors solve the critical engineering problem of controlling temperature and ensuring uniform contact in reactions involving solids. Their unparalleled heat transfer capabilities are their primary advantage, but this comes with the engineering trade-offs of particle erosion and complex fluid dynamics.

From Static Solids to a Fluid-Like State
To understand the value of this technology, we must first visualize how a static pile of granular material can be made to behave like a liquid.
The Anatomy of a Basic Reactor
Imagine a vertical cylinder containing a bed of fine solid particles, such as sand or a catalyst. At the bottom of this cylinder is a porous plate, known as a distributor, which allows a fluid (typically a gas) to be pumped upward through the solid bed.
The Principle of Fluidization
At a very low gas flow rate, the gas simply percolates through the spaces between the stationary particles. This is known as a packed bed or fixed bed.
As the gas velocity increases, it exerts a drag force on each particle. A critical point is reached where this upward drag force exactly balances the downward force of gravity on the particles. This is the minimum fluidization velocity.
Beyond this velocity, the bed expands and the particles become suspended in the upward-flowing gas. They begin to move around rapidly and randomly, creating a turbulent, thoroughly mixed system. The bed is now fluidized.
The "Boiling Liquid" Analogy
A fully fluidized bed exhibits remarkable fluid-like properties. It will flow under a pressure gradient, it has a discernible surface level like a liquid in a tank, and objects with a lower density than the bed will float on its surface. This vigorous mixing is the key to its advantages.
The Core Advantages of Fluidization
Engineers choose fluidized beds to solve specific, difficult challenges that other reactor types handle poorly. The primary benefits stem directly from the intense mixing of the solid particles.
Unmatched Temperature Uniformity
The rapid circulation of solids ensures that heat is distributed almost instantaneously throughout the reactor. This creates a nearly isothermal (uniform temperature) environment.
This feature is critical for highly exothermic (heat-releasing) reactions. Fluidized beds prevent the formation of dangerous "hot spots" that could damage the catalyst, reduce product selectivity, or even cause a runaway reaction.
Superior Heat and Mass Transfer
The turbulent motion and the enormous surface area of the suspended particles promote excellent contact between the reactant gas and the solid catalyst. This leads to very high rates of heat and mass transfer, which can significantly increase the overall reaction speed and efficiency.
Seamless Continuous Operation
Because the solids behave like a fluid, they can be continuously withdrawn and added back to the reactor. This is a massive advantage for processes where the catalyst deactivates quickly.
The classic example is Fluid Catalytic Cracking (FCC), where the catalyst is continuously removed, regenerated in a separate vessel (by burning off coke deposits), and then returned to the main reactor.
Understanding the Trade-offs and Challenges
Despite their advantages, fluidized beds are not a universal solution. Their dynamic nature introduces significant engineering challenges.
Particle Attrition and Elutriation
The constant, violent collisions between particles cause them to wear down and break apart, a process known as attrition. This creates fine dust that can be carried out of the reactor by the flowing gas.
This loss of material, called elutriation, requires the use of downstream equipment like cyclones to capture the fine particles and return them to the bed or dispose of them.
Reactor Erosion
The abrasive nature of the fast-moving solid particles can cause significant wear and tear on the reactor's internal walls, pipes, and measurement instruments. This requires the use of hardened materials and adds to maintenance costs.
Complex Hydrodynamics
The fluid mechanics of a fluidized bed are not simple. Gas tends to coalesce into large bubbles that can travel up through the bed, bypassing much of the solid catalyst. This bubbling or channeling reduces contact efficiency and can lower the overall reactant conversion.
The Issue of Back-Mixing
The intense mixing that provides uniform temperature also means the reactor's contents are well-mixed, similar to a Continuous Stirred-Tank Reactor (CSTR). This constant back-mixing can be a disadvantage for reactions that require a high concentration of reactants to proceed efficiently, which is better provided by a fixed-bed (or plug flow) reactor.
Making the Right Choice for Your Goal
The decision to use a fluidized bed reactor is a classic engineering trade-off between thermal control, continuous operation, and mechanical complexity.
- If your primary focus is managing a highly exothermic reaction: A fluidized bed is often the superior choice due to its exceptional temperature control, preventing catalyst damage and ensuring safety.
- If your primary focus is achieving the highest possible reactant conversion in a single pass: A fixed-bed (plug flow) reactor might be more suitable, as it avoids the back-mixing that dilutes reactant concentration.
- If your process involves a catalyst that deactivates quickly: The ability to continuously circulate and regenerate solids makes a fluidized bed system uniquely advantageous and often the only viable option.
Understanding these core principles allows you to select the most effective reactor technology for your specific chemical process.
Summary Table:
| Feature | Fluidized Bed Reactor | Fixed Bed Reactor |
|---|---|---|
| Temperature Control | Excellent (Isothermal) | Risk of Hot Spots |
| Heat/Mass Transfer | Superior | Limited |
| Operation Mode | Continuous Catalyst Regeneration | Batch/Semi-Batch |
| Solids Handling | Ideal for Fast-Deactivating Catalysts | Challenging |
| Mechanical Wear | High (Erosion, Attrition) | Low |
| Reactant Conversion | Lower (Due to Back-Mixing) | Higher (Plug Flow) |
Optimize your chemical processes with KINTEK's expertise in reactor technology.
Whether you are developing a new process or scaling up an existing one, selecting the right reactor is critical to your success. Fluidized bed reactors are powerful tools for managing exothermic reactions and enabling continuous operation with catalyst regeneration.
At KINTEK, we specialize in providing the high-quality lab equipment and consumables you need to test, develop, and perfect your reactions. Our team can help you navigate the complexities of reactor selection to achieve your goals for efficiency, safety, and yield.
Contact our experts today to discuss how we can support your laboratory's specific needs in chemical processing and catalyst research.
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
People Also Ask
- Why are sealed laboratory reaction vessels necessary in the hydrothermal synthesis of zeolites? Ensure Purity and Yield
- What is the function of a constant temperature hydrothermal reactor? Master Coal Fly Ash Activation
- What roles do autoclaves play in MFI zeolite synthesis? Master Hydrothermal Crystalline Growth
- What is the purpose of using a high-temperature hydrothermal reactor? Enhance Iodine@Activated Carbon Cathode Synthesis
- What role does an autoclave play in simulating PWR conditions? Advanced Material Validation for Nuclear Safety



















