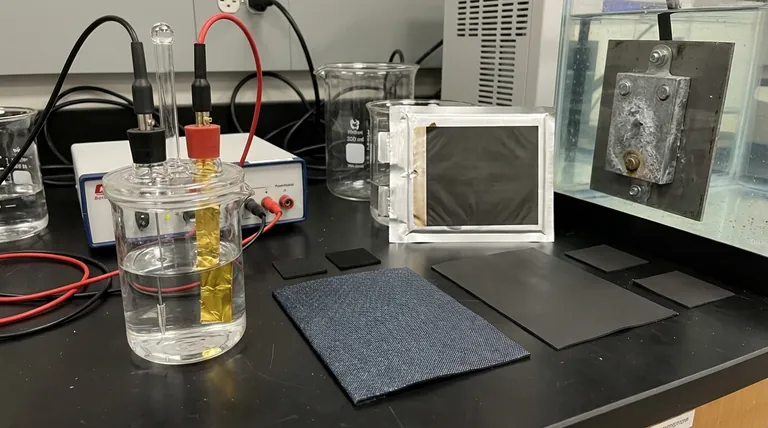
In many laboratory settings, the most commonly used anode materials are platinum, gold, and carbon (often as graphite or glassy carbon). These materials are chosen for their chemical inertness and electrical conductivity, ensuring they facilitate a reaction without interfering with it. However, this is only one piece of a much larger picture.
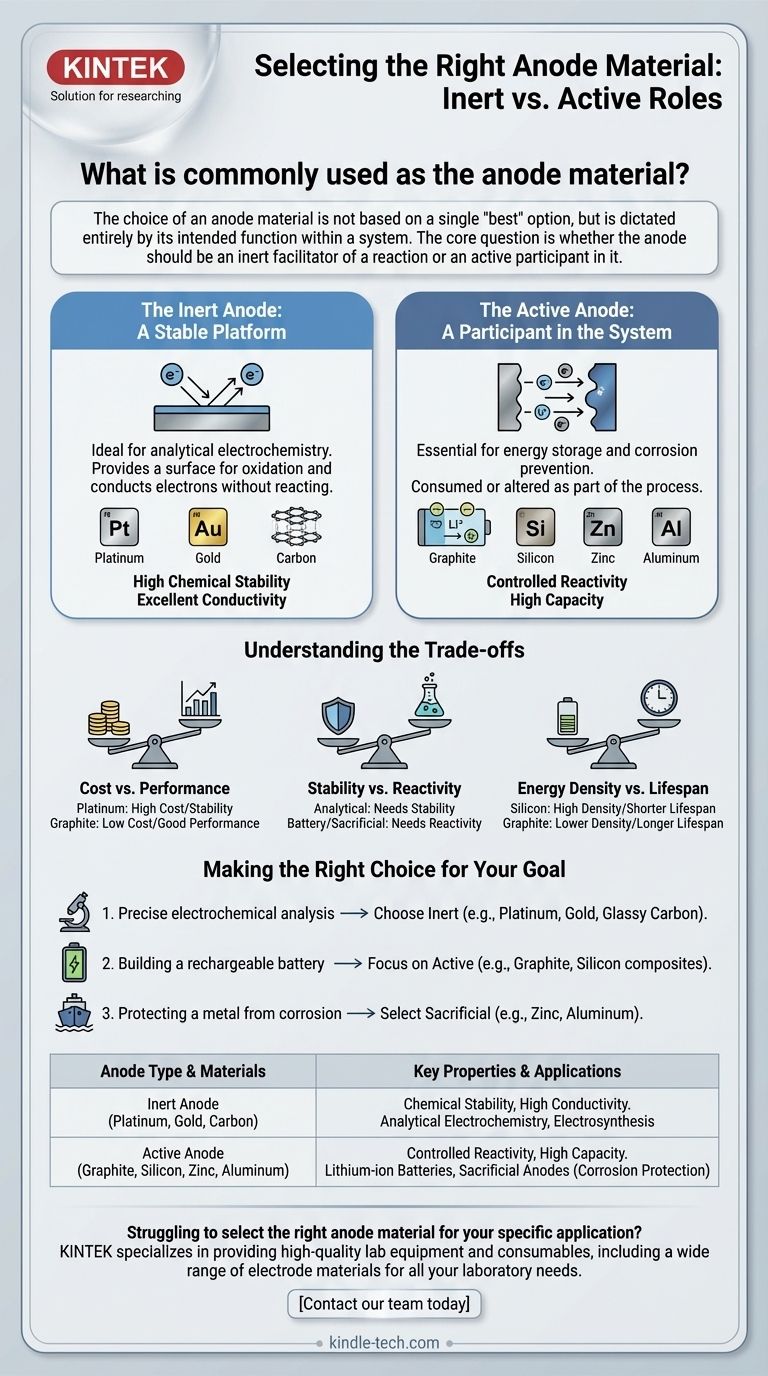
The choice of an anode material is not based on a single "best" option, but is dictated entirely by its intended function within a system. The core question is whether the anode should be an inert facilitator of a reaction or an active participant in it.
The Two Fundamental Roles of an Anode
The term "anode" simply refers to the electrode where oxidation (the loss of electrons) occurs. The ideal material for this role changes dramatically depending on the application's goal. We can separate these applications into two main categories: those requiring an inert anode and those requiring an active one.
The Inert Anode: A Stable Platform
In applications like analytical electrochemistry, an inert anode is essential. Its only job is to provide a surface for oxidation to occur and to conduct electrons out of the system.
The material itself should not change or react. This ensures that the measurements taken reflect the chemistry of the solution, not the degradation of the electrode.
This is why materials like platinum, gold, and carbon are standard choices. They possess the critical properties of high conductivity and exceptional chemical stability across a wide range of conditions.
The Active Anode: A Participant in the System
In many other critical technologies, the anode is designed to be an active and essential participant in the chemical process. Here, the material is consumed or altered as part of the system's function.
This is most common in energy storage and corrosion prevention. The material is chosen specifically for its reactive properties.
A prime example is a lithium-ion battery, where the anode is typically graphite. The graphite's job is to absorb and release lithium ions during charging and discharging. Its chemical reactivity is its primary feature.
Another key example is in corrosion prevention, where a sacrificial anode made of zinc, aluminum, or magnesium is attached to a steel structure like a ship's hull. The more reactive zinc corrodes (oxidizes) first, sacrificing itself to protect the steel.
Understanding the Trade-offs
Selecting an anode material always involves balancing competing factors. There is no single material that is perfect for every situation.
Cost vs. Performance
Platinum offers outstanding stability and catalytic properties but is extremely expensive. Graphite and other forms of carbon offer excellent performance for many applications at a fraction of the cost, making them ubiquitous in commercial products.
Stability vs. Reactivity
This is the central trade-off. For an analytical measurement, you need maximum stability so the anode doesn't interfere. For a battery or a sacrificial system, you need precisely controlled reactivity for the device to function.
Energy Density vs. Lifespan
In battery technology, this is a critical challenge. Silicon is being heavily researched as a next-generation anode material because it can hold significantly more lithium ions than graphite. However, it physically swells and shrinks dramatically during charging and discharging, which can cause it to degrade and fail quickly.
Making the Right Choice for Your Goal
The right anode is the one that serves the specific purpose of your electrochemical system. Your primary objective will immediately narrow the options.
- If your primary focus is precise electrochemical analysis: Choose an inert material like platinum, gold, or glassy carbon to ensure your measurements are not influenced by the electrode itself.
- If your primary focus is building a rechargeable battery: Focus on active materials with high capacity and cycling stability, such as graphite or emerging materials like silicon composites.
- If your primary focus is protecting a metal from corrosion: Select a sacrificial material that is more electrochemically active than the metal you are protecting, such as zinc or aluminum for steel.
Ultimately, understanding the anode's role—whether as a stable stage or an active participant—is the key to selecting the correct material for the task.
Summary Table:
| Anode Type | Common Materials | Key Properties | Primary Applications |
|---|---|---|---|
| Inert Anode | Platinum, Gold, Carbon (Graphite, Glassy Carbon) | Chemical Stability, High Conductivity | Analytical Electrochemistry, Electrosynthesis |
| Active Anode | Graphite, Silicon, Zinc, Aluminum | Controlled Reactivity, High Capacity | Lithium-ion Batteries, Sacrificial Anodes (Corrosion Protection) |
Struggling to select the right anode material for your specific application? KINTEK specializes in providing high-quality lab equipment and consumables, including a wide range of electrode materials for all your laboratory needs. Whether you require inert electrodes for precise analysis or are developing next-generation battery technology, our experts can help you find the optimal solution. Contact our team today to discuss your project and enhance your lab's capabilities!
Visual Guide
Related Products
- Conductive Carbon Cloth Carbon Paper Carbon Felt for Electrodes and Batteries
- Electrode Polishing Material for Electrochemical Experiments
- Custom PTFE Teflon Parts Manufacturer for PTFE Containers
- Conductive Boron Nitride BN Ceramics Composite for Advanced Applications
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
People Also Ask
- What are the material properties of carbon paper? Unlocking High Conductivity & Porosity for Your Lab
- What is the ideal operating environment for a glassy carbon sheet? Ensure Optimal Performance and Longevity
- What are 3 products that carbon nanotubes can be used in? Enhancing Batteries, Tires, and Composites
- Why are high surface area materials preferred for BES anodes? Maximize Microbial Power and Efficiency
- What are the common applications for carbon cloth? Unlock Its Potential in Energy & Electrochemical Systems
















