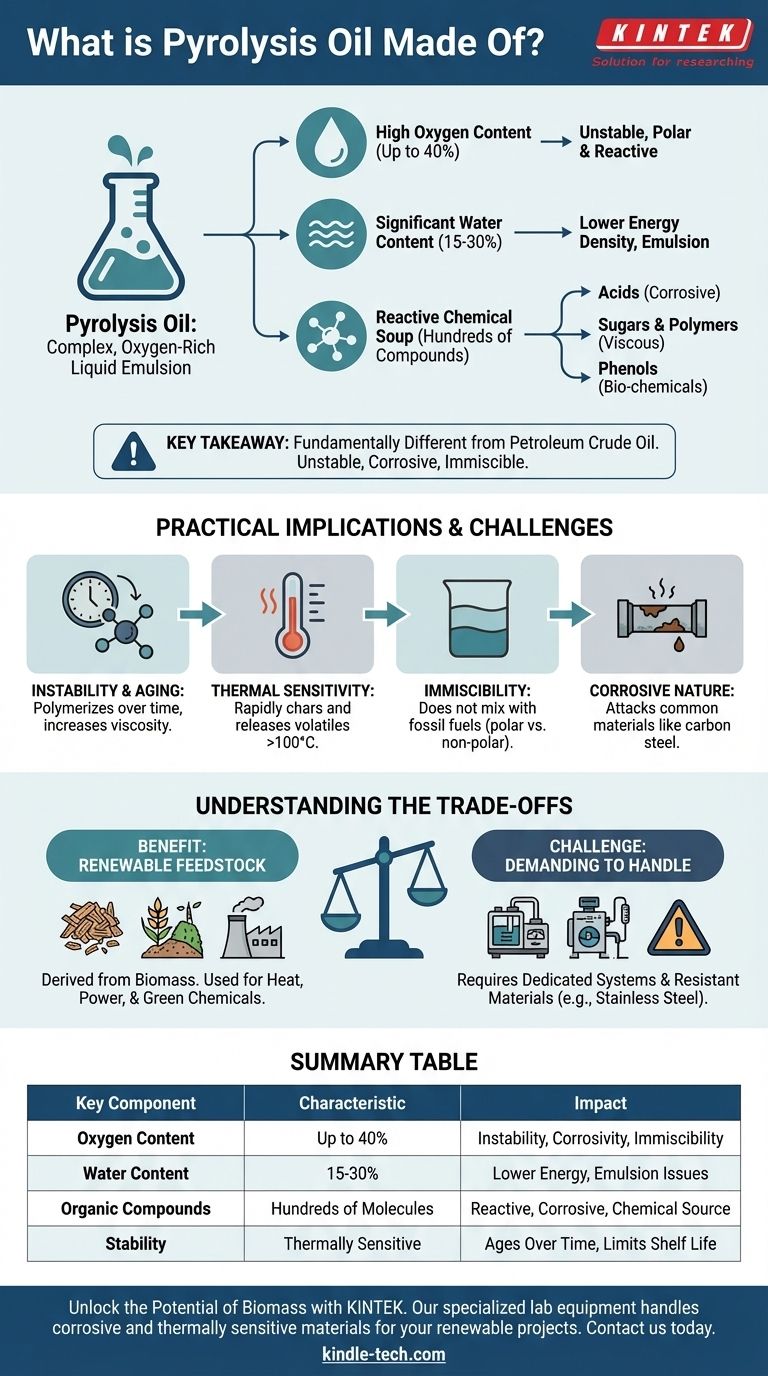
At its core, pyrolysis oil is a complex, oxygen-rich liquid emulsion. It is not a single chemical but a dense mixture composed of water and hundreds of different organic compounds, including acids, sugars, polymers, and phenols. This unique composition is a direct result of its production from the thermal decomposition of biomass in the absence of oxygen.
The most critical takeaway is that pyrolysis oil is fundamentally different from petroleum crude oil. Its high oxygen content makes it unstable, corrosive, and immiscible with fossil fuels, defining both its potential as a renewable resource and the significant challenges in its use and storage.

The Chemical Anatomy of Pyrolysis Oil
To understand pyrolysis oil, you must look at its composition not as a simple list of ingredients, but as a reactive chemical soup. Its properties are an emergent result of the interaction between its hundreds of components.
A Complex Oxygenated Mixture
The defining characteristic of pyrolysis oil is its high oxygen content, which can be up to 40% by weight. This is in stark contrast to petroleum crude, which has very little oxygen. This oxygen is bound within the various organic molecules and is the primary reason for the oil's unique behavior.
Significant Water Content
Pyrolysis oil is an emulsion, meaning it contains a substantial amount of water, often between 15-30%. This water is a byproduct of the pyrolysis process itself and is finely dispersed throughout the oil, contributing to its instability and lower energy density compared to fossil fuels.
A Spectrum of Chemical Families
The oil contains a wide range of molecules, from very simple to very complex.
- Low Molecular Weight Compounds: These include simple organic acids like acetic acid and aldehydes like formaldehyde. These components are largely responsible for the oil's low pH and corrosivity.
- High Molecular Weight Compounds: This group includes larger, more complex molecules like phenols and oligosaccharides (chains of sugar molecules), which are fragments of the original biomass structure (lignin and cellulose). These heavier compounds contribute to the oil's high viscosity.
Why Its Composition Matters: Practical Implications
The unique chemical makeup of pyrolysis oil directly dictates how it can be handled, stored, and used. Its properties are a world away from traditional fuels.
Instability is its Nature
Pyrolysis oil is made of intermediate, reactive decomposition products. These molecules are not fully stable and will continue to react with each other over time. This aging process leads to a gradual increase in viscosity as molecules combine (polymerize), and can eventually cause the oil to separate into different phases.
Thermal Sensitivity
Heating the oil accelerates these reactions dramatically. When heated to around 100°C or more, pyrolysis oil can rapidly polymerize, producing a solid char-like residue and releasing volatile organic compounds. This makes it challenging to use in systems that require pre-heating.
Immiscibility with Fossil Fuels
The high oxygen content makes the molecules in pyrolysis oil polar, similar to water. Conventional fossil fuels are non-polar. Just like oil and water, pyrolysis oil and petroleum products do not mix. This prevents it from being co-processed in existing oil refineries without significant pre-treatment.
Corrosive Properties
The presence of acetic acid and other organic acids makes pyrolysis oil corrosive to common materials like carbon steel. Utilizing it requires equipment made from more resistant materials, such as stainless steel, which adds to infrastructure costs.
Understanding the Trade-offs
Viewing pyrolysis oil requires balancing its renewable origins against its challenging chemical properties.
The Benefit: A Renewable Liquid Feedstock
The primary advantage is that it is a liquid produced directly from renewable biomass like wood, agricultural waste, or manure. It can be burned in specially designed boilers and furnaces for heat and power, or it can be seen as a potential source for extracting valuable bio-based chemicals.
The Challenge: A Demanding Product to Handle
The reality is that pyrolysis oil is a difficult fluid. Its instability limits its shelf life, its corrosivity demands specialized equipment, and its incompatibility with fossil fuels isolates it from existing infrastructure. It is not a "drop-in" fuel but a unique chemical product that requires a dedicated system.
Making the Right Choice for Your Goal
How you approach pyrolysis oil depends entirely on your objective. It is a substance of compromises, where its benefits in one area are often linked to challenges in another.
- If your primary focus is a direct drop-in fuel for existing engines or refineries: Pyrolysis oil is fundamentally unsuitable without extensive, and often expensive, upgrading to remove oxygen and improve stability.
- If your primary focus is a renewable fuel for dedicated industrial systems: It can be a viable option for new or retrofitted boilers and furnaces specifically designed to handle its corrosive nature and thermal sensitivity.
- If your primary focus is a source for green chemicals: Its rich composition of phenols, furans, and other organic compounds makes it a promising, though complex, feedstock for a future bio-based chemical industry.
Ultimately, understanding the reactive and oxygenated nature of pyrolysis oil is the key to correctly assessing its potential and its limitations.
Summary Table:
| Key Component | Characteristic | Impact |
|---|---|---|
| Oxygen Content | Up to 40% by weight | Causes instability, corrosivity, and immiscibility with fossil fuels |
| Water Content | 15-30% | Lowers energy density and contributes to emulsion instability |
| Organic Compounds | Hundreds of molecules (acids, sugars, phenols) | Makes the oil reactive, corrosive, and a potential source for bio-chemicals |
| Stability | Thermally sensitive, ages over time | Requires careful handling and limits shelf life |
Unlock the Potential of Biomass with KINTEK
Navigating the complexities of pyrolysis oil requires the right equipment and expertise. Whether you're processing biomass for renewable fuel or extracting valuable bio-chemicals, KINTEK's specialized lab equipment is designed to handle corrosive and thermally sensitive materials like pyrolysis oil.
We provide robust solutions for your laboratory needs, ensuring safe and efficient operations. Contact us today to discuss how our products can support your biomass conversion projects and help you achieve your renewable energy goals.
Get in touch with our experts now!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Laboratory Hybrid Tissue Grinding Mill
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success











