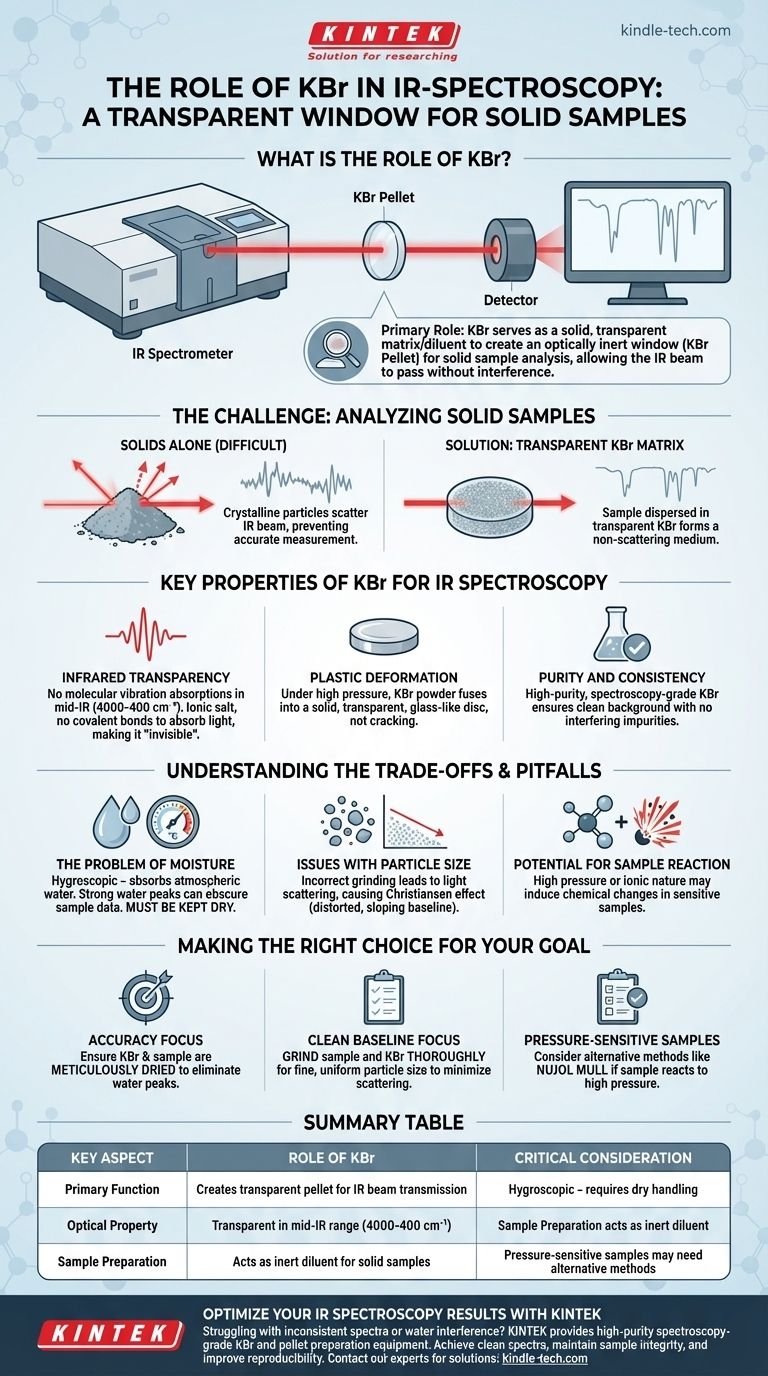
In infrared (IR) spectroscopy, potassium bromide (KBr) serves as a solid matrix or diluent used to hold a solid sample for analysis. Its primary role is to create a small, transparent window—called a KBr pellet—through which the instrument's IR beam can pass. The solid sample is finely ground and mixed with pure KBr powder, then compressed under high pressure to form this pellet.
The core function of KBr is to provide an optically inert environment for a solid sample. Because KBr is transparent to infrared radiation, it allows the spectrometer to measure the absorption spectrum of the sample itself without interference from the surrounding matrix.

The Fundamental Challenge: Analyzing Solid Samples
IR spectroscopy relies on measuring how a sample's chemical bonds absorb infrared light. For this to work, the IR beam must be able to pass through the sample cleanly.
Why Solids are Difficult
Unlike liquids or gases which can be placed in simple transparent cells, a solid powder cannot be analyzed directly. The crystalline particles would scatter the IR beam, preventing an accurate absorption measurement.
The Solution: A Transparent Matrix
To overcome this, the solid sample is dispersed evenly within a medium that does not interfere with the measurement. This medium must be transparent in the IR region and form a solid, non-scattering medium when prepared. This is precisely the role KBr fulfills.
Key Properties of KBr for IR Spectroscopy
KBr is not the only material that can be used, but it is the most common due to its ideal combination of physical and optical properties.
Infrared Transparency
The most critical property of KBr is its lack of molecular vibration absorptions in the mid-infrared range (4000-400 cm⁻¹). Because it is a simple ionic salt, it has no covalent bonds that absorb IR light in this region, making it effectively invisible to the spectrometer and creating a clear window for the sample's spectrum.
Plastic Deformation
When subjected to high pressure (on the order of tons per square inch), KBr powder has the unique property of plastic flow. This means it fuses together to form a solid, transparent, and glass-like disc rather than cracking or crumbling.
Purity and Consistency
High-purity, spectroscopy-grade KBr is readily available. This ensures that no impurities are present that might introduce unwanted peaks into the spectrum, providing a clean and reliable background.
Understanding the Trade-offs and Pitfalls
While the KBr pellet technique is powerful, it is not without challenges. Proper preparation is essential for obtaining a high-quality spectrum.
The Problem of Moisture
KBr is hygroscopic, meaning it readily absorbs water from the atmosphere. Water has very strong, broad IR absorption bands that can easily obscure important peaks from the sample. Therefore, the KBr must be kept perfectly dry, often by storing it in a desiccator or oven.
Issues with Particle Size
If the sample is not ground finely enough or mixed homogeneously with the KBr, its particles will scatter the IR light. This leads to a distorted, sloping baseline and reduced spectral quality, an issue known as the Christiansen effect.
Potential for Sample Reaction
The extremely high pressure used to form the pellet can sometimes induce a chemical reaction or a phase change in the sample. Additionally, the ionic nature of KBr can interact with certain types of samples, altering their spectra.
Making the Right Choice for Your Goal
Proper technique is paramount when using KBr to ensure your results are accurate and reproducible.
- If your primary focus is accuracy: Ensure both your KBr and sample are meticulously dried before preparation to eliminate interfering water peaks.
- If your primary focus is a clean baseline: Grind the sample and KBr together thoroughly to achieve a fine, uniform particle size, which minimizes light scattering.
- If you suspect your sample is pressure-sensitive: Consider an alternative solid sampling method, such as preparing a Nujol mull, which does not require high pressure.
Ultimately, understanding KBr's role as an inert optical window is the key to mastering this fundamental spectroscopic technique.
Summary Table:
| Key Aspect | Role of KBr |
|---|---|
| Primary Function | Creates transparent pellet for IR beam transmission |
| Optical Property | Transparent in mid-IR range (4000-400 cm⁻¹) |
| Sample Preparation | Acts as inert diluent for solid samples |
| Critical Consideration | Hygroscopic - requires dry handling |
| Alternative Use Cases | Pressure-sensitive samples may need alternative methods |
Optimize Your IR Spectroscopy Results with KINTEK
Are you struggling with inconsistent IR spectra or water interference in your solid sample analysis? KINTEK specializes in high-purity laboratory equipment and consumables that ensure accurate spectroscopic results.
Our spectroscopy-grade KBr and pellet preparation equipment help you:
- Achieve clean, interference-free spectra with optimal transparency
- Maintain sample integrity with proper drying and handling solutions
- Improve reproducibility with consistent, high-quality materials
Whether you're analyzing pharmaceuticals, polymers, or research samples, KINTEK provides the reliable lab equipment and consumables your laboratory needs for precise IR spectroscopy.
Contact our experts today to discuss your specific application requirements and discover how our solutions can enhance your analytical capabilities!
Visual Guide
Related Products
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- kbr pellet press 2t
- Laboratory Hydraulic Press Lab Pellet Press Machine for Glove Box
People Also Ask
- What role does a laboratory hydraulic press play in the preparation of solid electrolyte pellets? Ensure Data Accuracy
- How hot is a hydraulic press? Understanding the Critical Heat in Your Hydraulic System
- What is the pressed powder pellet method? A Guide to Accurate FTIR Sample Preparation
- Why use KBr for IR? Achieve Clear, Unobstructed Spectra for Solid Samples
- What is the pellet technique in IR? Master Solid Sample Preparation for Clear Spectroscopy



















