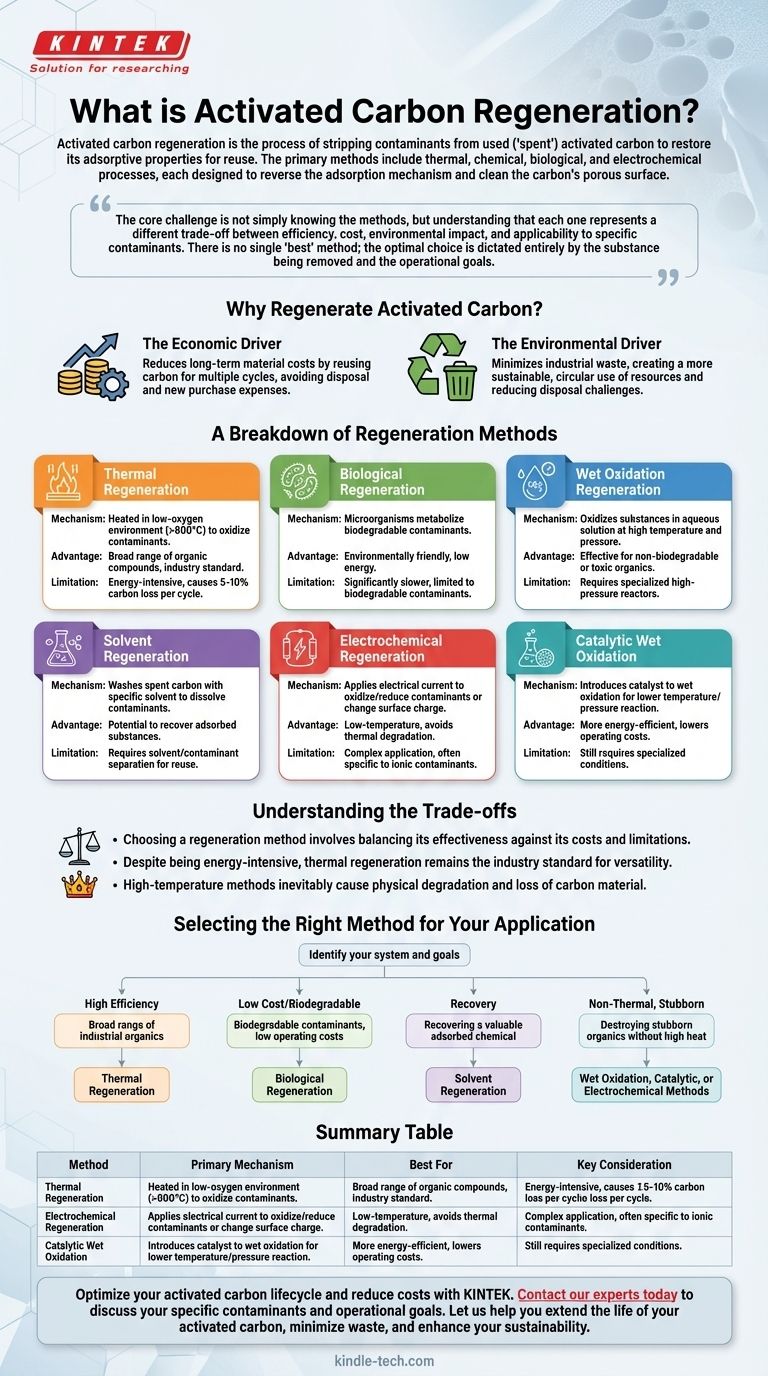
Activated carbon regeneration is the process of stripping contaminants from used, or "spent," activated carbon to restore its adsorptive properties for reuse. The primary methods include thermal, chemical, biological, and electrochemical processes, each designed to reverse the adsorption mechanism and clean the carbon's porous surface.
The core challenge is not simply knowing the methods, but understanding that each one represents a different trade-off between efficiency, cost, environmental impact, and applicability to specific contaminants. There is no single "best" method; the optimal choice is dictated entirely by the substance being removed and the operational goals.

Why Regenerate Activated Carbon?
Activated carbon has a finite capacity. Once its vast network of pores is filled with adsorbed contaminants, it becomes ineffective and must be replaced or regenerated.
The Economic Driver
Disposing of spent carbon and purchasing fresh material is a significant operational expense. Regeneration allows a single batch of activated carbon to be used for multiple cycles, drastically reducing long-term material costs.
The Environmental Driver
Spent activated carbon, laden with potentially hazardous materials, can be classified as industrial waste, creating disposal challenges. Regeneration minimizes waste and promotes a more sustainable, circular use of resources.
A Breakdown of Regeneration Methods
The method chosen depends almost entirely on the nature of the bond between the contaminant (adsorbate) and the carbon surface.
Thermal Regeneration
This is the most common and robust method. The spent carbon is heated in a controlled, low-oxygen environment to temperatures typically exceeding 800°C (1500°F).
This intense heat volatilizes and then thermally destroys (oxidizes) the adsorbed organic contaminants, effectively cleaning the carbon pores. It is highly effective for a broad range of organic compounds.
Biological Regeneration
This method uses microorganisms to break down and metabolize biodegradable contaminants adsorbed onto the carbon. It is an environmentally friendly, low-energy process.
However, it is significantly slower than thermal methods and is only effective for contaminants that are readily biodegradable.
Wet Oxidation Regeneration
In this process, adsorbed substances are oxidized into simpler compounds in an aqueous solution at high temperatures and pressures. An oxidizing agent, such as oxygen or air, is used.
This method is effective for regenerating carbon spent on non-biodegradable or toxic organic compounds, but it requires specialized high-pressure reactors.
Solvent Regeneration
This technique involves washing the spent carbon with a specific solvent that can dissolve the adsorbed contaminants, effectively pulling them out of the carbon's pores.
A key advantage is the potential to recover the adsorbed substance, which can be valuable. The main challenge then becomes separating the contaminant from the solvent for reuse.
Electrochemical Regeneration
This method applies an electrical current to the spent carbon. The process can work in two ways: by directly oxidizing or reducing the contaminant into a less adsorbable form, or by changing the carbon's surface charge to repel the adsorbed molecules.
It is a low-temperature process that avoids the thermal degradation of the carbon, but its application can be complex and is often specific to certain ionic contaminants.
Catalytic Wet Oxidation Method
This is an advanced form of wet oxidation. It introduces a catalyst to the process, which allows the oxidation reaction to occur at lower temperatures and pressures.
Using a catalyst makes the process more energy-efficient and can reduce the severity of the operating conditions required, lowering capital and operating costs compared to standard wet oxidation.
Understanding the Trade-offs
Choosing a regeneration method involves balancing its effectiveness against its costs and limitations. No single method is universally superior.
The Dominance of Thermal Methods
Despite being energy-intensive, thermal regeneration remains the industry standard. Its ability to effectively destroy a wide array of organic contaminants makes it the most versatile and reliable option for many large-scale water and air purification applications.
The Carbon Loss Factor
High-temperature methods like thermal regeneration inevitably cause some physical degradation and loss of the activated carbon material itself (typically 5-10% per cycle). This means the carbon cannot be regenerated indefinitely and will eventually need to be replaced.
The Specificity of Non-Thermal Methods
Methods like solvent, biological, and electrochemical regeneration are highly specialized. Their success hinges on a favorable chemistry between the contaminant, the carbon, and the regeneration agent. They are powerful in the right niche but lack the universal applicability of thermal treatment.
Selecting the Right Method for Your Application
Your final decision should be based on a clear analysis of your specific system and goals.
- If your primary focus is high efficiency for a broad range of industrial organics: Thermal regeneration is the most established and versatile solution.
- If your primary focus is treating biodegradable contaminants with low operating costs: Biological regeneration offers a sustainable and energy-efficient pathway.
- If your primary focus is recovering a valuable adsorbed chemical: Solvent regeneration is the only practical method that allows for contaminant retrieval.
- If your primary focus is destroying stubborn organic compounds without high heat: Wet oxidation, catalytic oxidation, or electrochemical methods provide powerful, albeit more complex, alternatives.
Ultimately, selecting the right regeneration process is a strategic decision that directly impacts your operational costs, efficiency, and environmental footprint.
Summary Table:
| Method | Primary Mechanism | Best For | Key Consideration |
|---|---|---|---|
| Thermal Regeneration | High-temperature oxidation (>800°C) | Broad range of industrial organics | Industry standard; causes 5-10% carbon loss per cycle |
| Biological Regeneration | Microorganism metabolism | Biodegradable contaminants | Low-energy but slow process |
| Solvent Regeneration | Chemical washing with solvent | Recovering valuable adsorbed chemicals | Requires solvent/contaminant separation |
| Wet Oxidation | Oxidation in aqueous solution | Stubborn, non-biodegradable organics | Requires high-pressure reactors |
| Electrochemical | Electrical current application | Specific ionic contaminants | Low-temperature; avoids thermal degradation |
Optimize your activated carbon lifecycle and reduce costs with KINTEK.
Choosing the right regeneration method is critical for your lab's efficiency and budget. Whether you're processing industrial organics, recovering valuable chemicals, or treating biodegradable waste, KINTEK's expertise in lab equipment and consumables can help you select and implement the ideal solution.
Contact our experts today to discuss your specific contaminants and operational goals. Let us help you extend the life of your activated carbon, minimize waste, and enhance your sustainability.
Visual Guide
Related Products
- 1200℃ Muffle Furnace Oven for Laboratory
- Graphite Vacuum Furnace Negative Material Graphitization Furnace
- Graphite Vacuum Furnace Bottom Discharge Graphitization Furnace for Carbon Materials
- Horizontal High Temperature Graphite Vacuum Graphitization Furnace
- Vacuum Heat Treat and Pressure Sintering Furnace for High Temperature Applications
People Also Ask
- Why KBr is used for making pellets to do IR measurements? Achieve Clear, Accurate Spectra
- How does a precision oven ensure epoxy and nanosheet coating quality? Achieve Perfect Cross-Linking and Bond Strength
- Does melting point ever change? Unlock the Secrets of Pressure and Purity
- What metals can you blacksmith with? Discover Forgeable Metals for Every Project
- What is the purpose of sputtering? Achieve Superior Thin-Film Coatings for Advanced Applications
- What is a microwave pyrolysis reactor? A Guide to Faster, More Efficient Thermal Processing
- How does graphite react to heat? Unlocking Its Unique High-Temperature Strengths
- What are the failures related to heat treating operations? Prevent Distortion, Cracking & Soft Spots



















