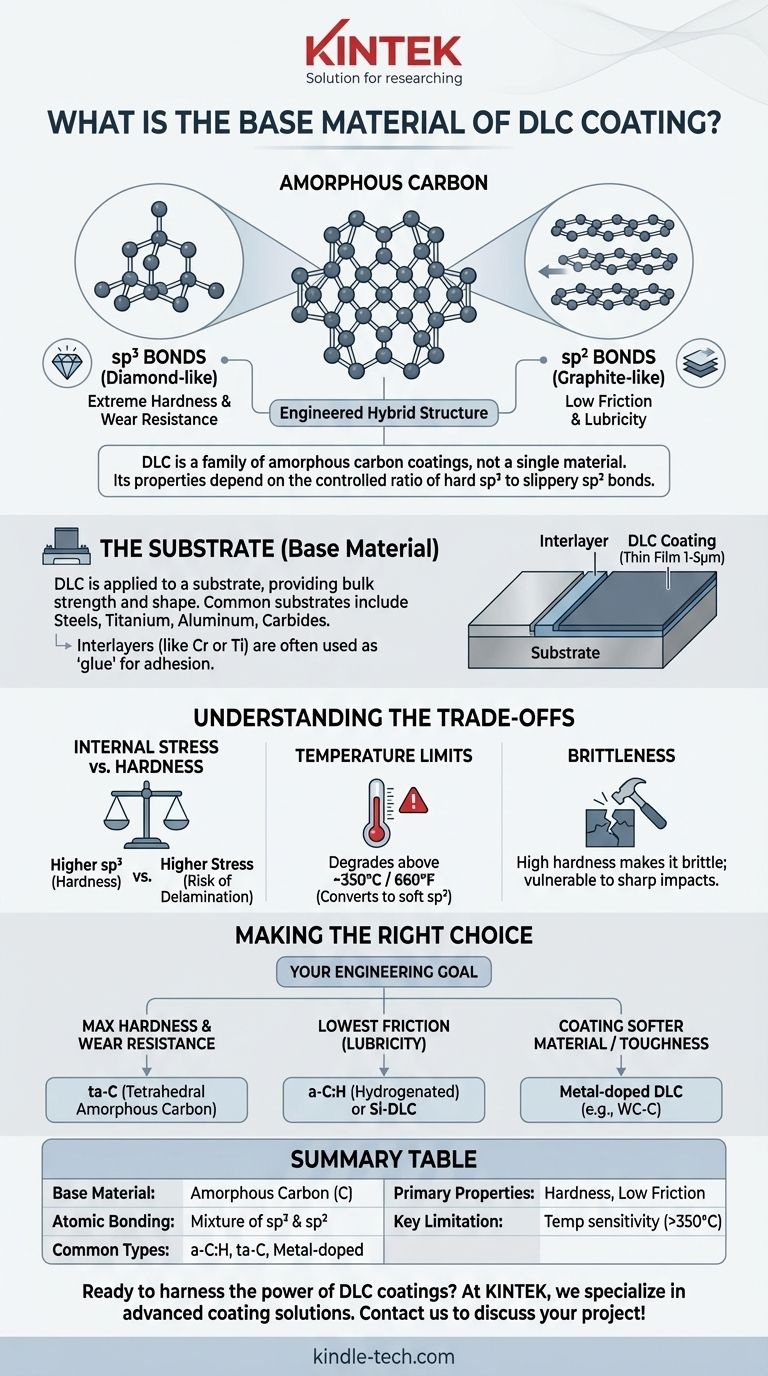
At its most fundamental level, the base material of a Diamond-Like Carbon (DLC) coating is amorphous carbon. This means it is composed of carbon atoms, but unlike diamond or graphite, they are arranged without a repeating crystalline structure. The unique properties of DLC arise from its hybrid nature, containing a mixture of both diamond-type and graphite-type atomic bonds.
At its core, DLC is not a single material but a family of amorphous carbon coatings. Its true value lies in the engineered mixture of diamond-like (sp³) and graphitic (sp²) atomic bonds, allowing for a unique combination of extreme hardness and low-friction lubricity.

What Does "Diamond-Like Carbon" Actually Mean?
To understand DLC, you must look beyond the element and focus on its atomic structure. The name itself describes a material that mimics the properties of diamond without being a true diamond.
The Core Ingredient: Carbon
The coating is made entirely of carbon atoms, the same element that forms both diamond (the hardest known natural material) and graphite (a soft, slippery lubricant). The difference lies entirely in how those atoms are bonded together.
The Hybrid Bond Structure: sp³ vs. sp²
This is the key to DLC's performance.
- sp³ Bonds: This is the tetrahedral bond found in natural diamond. It is incredibly strong and rigid, giving DLC its characteristic high hardness and wear resistance.
- sp² Bonds: This is the planar bond found in graphite. These bonds are weaker between their layers, allowing them to slide easily, which gives DLC its low coefficient of friction and lubricity.
DLC is an engineered film where the ratio of hard sp³ to slippery sp² bonds is carefully controlled during the deposition process to achieve specific properties.
The Role of Hydrogen
Many common forms of DLC are hydrogenated (designated as a-C:H). During the deposition process, hydrogen is introduced to help stabilize the amorphous structure. This reduces the high internal stresses that can build up in the film, improving adhesion and allowing for thicker coatings.
The "Base Material" It's Applied To (The Substrate)
While the coating itself is carbon, it is almost always applied to another material, known as the substrate. The choice of substrate is as critical as the coating itself.
Common Substrates
DLC can be applied to a vast range of materials, provided they are compatible with the vacuum deposition process. Common substrates include most steels, titanium alloys, aluminum alloys, carbides, and even some plastics and ceramics.
Why Substrate Choice Matters
The substrate provides the bulk strength and shape of the component. The DLC coating is a very thin film (typically 1-5 microns) that only provides the surface properties. A hard DLC coating on a soft substrate that deforms easily will simply crack and flake off.
The Need for Interlayers
Adhesion is a major consideration. Due to differences in material properties and internal stresses, DLC is often deposited onto one or more metallic interlayers. A thin layer of a material like chromium (Cr) or titanium (Ti) is often applied to the substrate first to act as a "glue," ensuring the DLC film adheres strongly.
Understanding the Trade-offs
No coating is perfect, and choosing DLC requires understanding its limitations. Being aware of these trade-offs is crucial for successful implementation.
Internal Stress vs. Hardness
A higher ratio of diamond-like sp³ bonds increases hardness but also dramatically increases the internal compressive stress of the coating. If not properly managed (e.g., with hydrogen or interlayers), this stress can cause the coating to delaminate or flake off the substrate.
Temperature Limitations
DLC is not suitable for high-temperature applications. When exposed to temperatures above roughly 350°C (660°F) in air, the hard sp³ bonds begin to break down and convert into softer sp² (graphitic) bonds, causing the coating to lose its hardness and protective qualities.
Brittleness and Impact Resistance
Because of its high hardness, DLC is inherently brittle. It provides excellent sliding wear resistance but can be chipped or fractured by sharp, direct impacts. The toughness of the underlying substrate plays a significant role in its overall impact durability.
Making the Right Choice for Your Goal
The term "DLC" represents a family of coatings. The correct choice depends entirely on your primary engineering objective.
- If your primary focus is maximum hardness and wear resistance: You need a coating with the highest possible sp³ content, such as tetrahedral amorphous carbon (ta-C), which is non-hydrogenated.
- If your primary focus is the lowest possible friction: A hydrogenated amorphous carbon (a-C:H) or silicon-doped DLC (Si-DLC) often provides the best lubricity, especially in humid or atmospheric conditions.
- If your focus is coating a softer material or improving toughness: A metal-doped DLC (e.g., WC-C), which incorporates tungsten carbide nanoparticles, can offer lower internal stress and better load-bearing support.
Understanding that DLC is an engineered form of carbon, not a monolithic substance, is the key to unlocking its full potential for your application.
Summary Table:
| Key Aspect | Description |
|---|---|
| Base Material | Amorphous Carbon (C) |
| Atomic Bonding | Mixture of diamond-like (sp³) and graphite-like (sp²) bonds |
| Common Types | Hydrogenated (a-C:H), Tetrahedral (ta-C), Metal-doped (e.g., WC-C) |
| Primary Properties | Extreme Hardness, Low Friction, Wear Resistance |
| Key Limitation | Temperature sensitivity (degrades above ~350°C / 660°F) |
Ready to harness the power of DLC coatings for your components?
At KINTEK, we specialize in advanced coating solutions for the laboratory and industrial sectors. Our expertise in DLC and other PVD coatings can help you achieve superior surface properties, from extreme wear resistance to low friction, tailored to your specific substrate and application requirements.
Contact us today to discuss your project and discover how our lab equipment and consumables can provide the perfect coating solution for your needs.
Get in touch with our experts now!
Visual Guide
Related Products
- Laboratory CVD Boron Doped Diamond Materials
- CVD Diamond Domes for Industrial and Scientific Applications
- CVD Diamond Cutting Tool Blanks for Precision Machining
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
People Also Ask
- What is the function of chemical vapor deposition (CVD) equipment? Precision Growth for BDD Electrodes
- What is the carbon footprint of diamond mining? Uncovering the True Environmental and Ethical Cost
- What are the three types of coating? A Guide to Architectural, Industrial, and Special Purpose
- How is something diamond coated? A Guide to CVD Growth vs. Plating Methods
- What physical conditions does an HPHT press provide for BDD synthesis? Achieve Extreme 5 GPa & 1800 K Conditions














