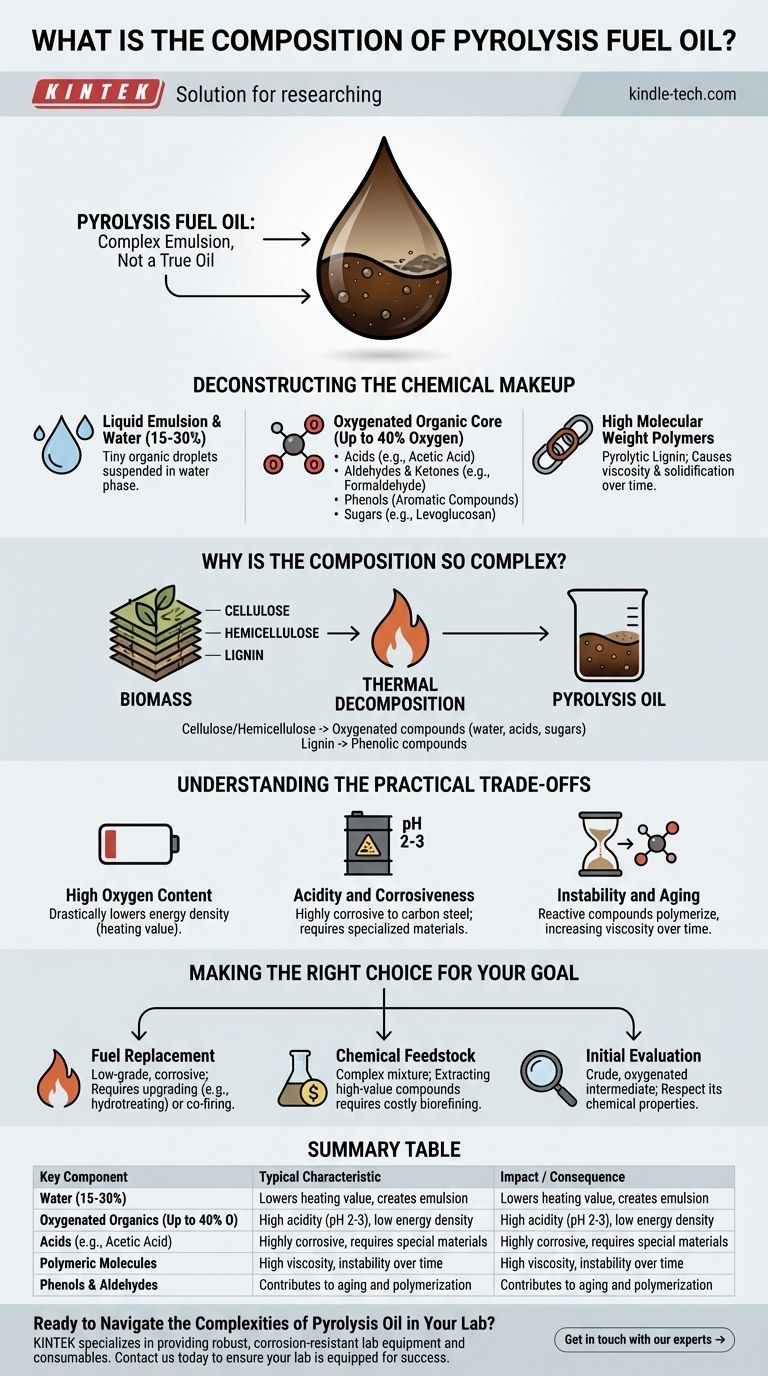
In short, pyrolysis fuel oil is not a true oil but a complex, dark brown liquid emulsion. It is primarily composed of hundreds of different oxygenated organic compounds, water, and larger polymeric molecules that are direct breakdown products of biomass.
Pyrolysis oil's unique and challenging composition is a direct reflection of its source material. Understanding that it is the deconstructed remnants of plant matter—specifically cellulose, hemicellulose, and lignin—is the key to grasping its properties and its potential.

Deconstructing the Chemical Makeup
A Liquid Emulsion, Not a Simple Oil
Pyrolysis oil is a micro-emulsion where tiny droplets of organic compounds are suspended in a water phase. A significant portion, often 15-30% by weight, is water that comes from both the original moisture in the biomass and from the chemical reactions during pyrolysis.
The Oxygenated Organic Core
Unlike petroleum-based fuels, which are almost entirely hydrocarbons, pyrolysis oil is rich in oxygen. This oxygen, which can be up to 40% of the oil's weight, is bound within its chemical structures.
These structures span a wide range of molecular families, including:
- Acids: Primarily low molecular weight compounds like acetic acid, which make the oil highly acidic.
- Aldehydes & Ketones: Reactive compounds such as formaldehyde and hydroxyacetone.
- Phenols: Aromatic compounds derived from the breakdown of lignin.
- Sugars: Includes levoglucosan and other oligosaccharides from the breakdown of cellulose.
High Molecular Weight Polymers
Not all of the biomass breaks down into small molecules. The process also creates larger, heavier molecules, often referred to as pyrolytic lignin or polymers. These contribute to the oil's high viscosity and its tendency to solidify over time.
Why Is the Composition So Complex?
The final composition of pyrolysis oil is a direct consequence of the thermal decomposition of the three main components of biomass.
Influence of Cellulose and H hemicellulose
These components are long chains of sugar molecules (carbohydrates). When heated rapidly without oxygen, they break apart into a variety of smaller oxygenated compounds, including water, acetic acid, and various sugars and aldehydes.
Influence of Lignin
Lignin is a complex aromatic polymer that gives plants their rigidity. Its thermal decomposition is the primary source of the phenolic compounds found in pyrolysis oil.
Understanding the Practical Trade-offs
The unique composition of pyrolysis oil presents significant challenges that are critical to understand for any practical application.
High Oxygen Content
The high oxygen content is the root of many of its properties. It drastically lowers the energy density (heating value) of the oil compared to conventional fossil fuels.
Acidity and Corrosiveness
The presence of acetic acid and other organic acids gives the oil a very low pH, typically between 2 and 3. This makes it highly corrosive to common construction materials like carbon steel, requiring specialized and more expensive handling equipment.
Instability and Aging
Many of the compounds in the oil, such as aldehydes and phenols, are reactive. Over time, even at room temperature, they can react with each other (polymerize), causing the oil's viscosity to increase and solids to form. This instability complicates long-term storage and transportation.
Making the Right Choice for Your Goal
Your approach to pyrolysis oil must be dictated by its fundamental chemistry and your specific objective.
- If your primary focus is direct fuel replacement: You must treat it as a low-grade, corrosive fuel that likely requires upgrading (e.g., hydrotreating to remove oxygen) or co-firing in specialized industrial boilers.
- If your primary focus is chemical feedstock: The value is in the complex mixture of chemicals, but extracting specific high-value compounds like phenols requires sophisticated and costly biorefinery separation processes.
- If your primary focus is simply initial evaluation: Do not mistake pyrolysis oil for a drop-in replacement for diesel or fuel oil. It is a crude, oxygenated intermediate product that demands respect for its chemical properties.
Understanding this complex composition is the first step toward unlocking the potential of pyrolysis oil while navigating its significant technical challenges.
Summary Table:
| Key Component | Typical Characteristic | Impact / Consequence |
|---|---|---|
| Water | 15-30% by weight | Lowers heating value, creates emulsion |
| Oxygenated Organics | Up to 40% oxygen content | High acidity (pH 2-3), low energy density |
| Acids (e.g., Acetic Acid) | Major component | Highly corrosive, requires special materials |
| Polymeric Molecules | From lignin/cellulose breakdown | High viscosity, instability over time |
| Phenols & Aldehydes | Reactive compounds | Contributes to aging and polymerization |
Ready to Navigate the Complexities of Pyrolysis Oil in Your Lab?
Understanding the unique composition of pyrolysis fuel oil is crucial for effective research, testing, and application development. Its corrosive nature and instability demand specialized lab equipment for accurate handling and analysis.
KINTEK specializes in providing robust, corrosion-resistant lab equipment and consumables tailored for challenging materials like pyrolysis oil. Whether you need reactors, storage solutions, or analytical tools, our products are designed to handle the demands of biomass-derived fuels safely and efficiently.
Contact us today to find the right equipment for your pyrolysis oil projects and ensure your lab is equipped for success. Get in touch with our experts →
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Custom PTFE Teflon Parts Manufacturer for PTFE Measuring Cylinder 10/50/100ml
- High Purity Zinc Foil for Battery Lab Applications
People Also Ask
- What is the difference between fast and flash pyrolysis? Maximize Your Bio-Oil Yield
- What is the disadvantage of pyrolysis? Key Economic and Technical Challenges Explained
- What are the advantages and disadvantages of rotary kiln incineration? A Guide to High-Temperature Waste Destruction
- What are the feedstocks used in pyrolysis? Unlock the Potential of Diverse Organic Materials
- What are the byproducts of the pyrolysis plant? Turn Waste into Bio-Oil, Bio-Char & Syngas
- What is different between calcination? Unlocking Thermal Processing for Material Science
- What is the end result of pyrolysis? Converting Waste into Bio-Char, Bio-Oil, and Syngas
- What is the carbon content of pyrolysis oil? A Deep Dive into Its Composition and Uses











