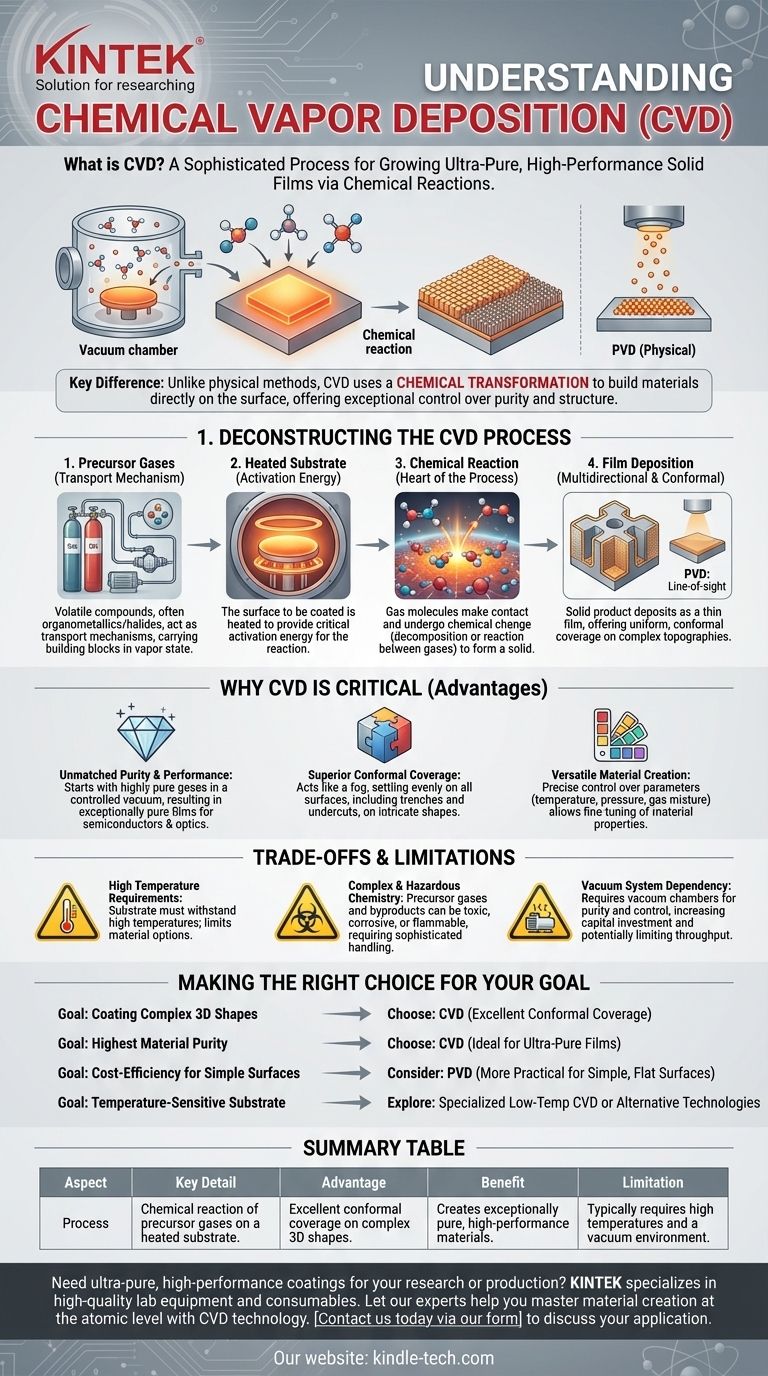
In essence, Chemical Vapor Deposition (CVD) is a sophisticated process for "growing" an ultra-pure, high-performance solid film on a surface using chemical reactions. Precursor gases are introduced into a chamber containing a heated object, known as a substrate. The heat energizes the gases, causing them to react or decompose and deposit a new solid material onto the substrate's surface, atom by atom or molecule by molecule.
Unlike physical methods that simply transfer a material from a source to a target, CVD's defining characteristic is its use of a chemical transformation. It builds a new material directly on the substrate, offering exceptional control over the final product's purity and structure.

Deconstructing the CVD Process
To truly understand CVD, we must look at its core components and sequence. The process is a carefully controlled chemical event happening within a specialized environment.
The Role of Precursor Gases
The process begins with one or more volatile precursor gases. These are compounds, often organometallics or halides, that contain the atoms needed for the final film.
These gases act as the transport mechanism, carrying the essential building blocks into the reaction chamber in a vapor state.
The Heated Substrate
The substrate is the object or surface that will be coated. It is heated to a specific, high temperature inside the reaction chamber.
This heat is not just for warmth; it provides the critical activation energy required to initiate the chemical reaction of the precursor gases on or near the substrate's surface.
The Chemical Reaction
This is the heart of the CVD process. Once the precursor gases make contact with the hot substrate, they undergo a chemical change.
This can be a decomposition, where a single gas breaks down into a solid and gaseous byproducts, or a reaction between multiple gases to form the desired solid.
The Resulting Film Deposition
The solid product of this chemical reaction deposits onto the substrate, forming a thin, solid film. This film can be crystalline, amorphous, or a combination of both.
Because the reactants are in a gas phase, the deposition is multidirectional and conformal, meaning it can uniformly coat complex, non-flat surfaces. This distinguishes it from line-of-sight techniques like Physical Vapor Deposition (PVD).
Why CVD is a Critical Manufacturing Technique
CVD is not used for its speed but for the exceptional quality and unique capabilities it enables. Its value lies in the properties of the materials it creates.
Unmatched Purity and Performance
Since the process begins with highly pure gases and occurs in a controlled vacuum environment, the resulting solid films are exceptionally pure and high-performance. This is critical for applications like semiconductors and advanced optics.
Superior Conformal Coverage
Imagine trying to paint a complex 3D object. A spray gun (like PVD) only coats what it can see. CVD acts more like a fog that settles evenly on every single surface, including trenches, holes, and undercuts. This ability to create uniform films on intricate topographies is a primary advantage.
Versatile Material Creation
By precisely adjusting the process parameters—such as temperature, pressure, and the mixture of precursor gases—engineers can fine-tune the material's properties. This versatility allows for the creation of films with specific physical, chemical, or electrical characteristics.
Understanding the Trade-offs and Limitations
No process is without its challenges. An objective assessment requires understanding CVD's inherent constraints.
High Temperature Requirements
The need for a heated substrate means the substrate material itself must be able to withstand high temperatures without deforming or degrading. This limits the types of materials that can be coated with standard CVD.
Complex and Hazardous Chemistry
The precursor gases and their reaction byproducts can be toxic, corrosive, or flammable. This necessitates sophisticated handling, safety, and exhaust management systems, increasing operational complexity and cost.
Vacuum System Dependency
Most CVD processes are performed under vacuum to ensure gas purity and control the reaction environment. Vacuum chambers and pumping systems represent a significant capital investment and can limit the throughput of the manufacturing process.
Making the Right Choice for Your Goal
Your decision to use or specify CVD should be driven by the end goal for your material or component.
- If your primary focus is coating complex 3D shapes: CVD is often the superior choice over line-of-sight methods due to its excellent conformal coverage.
- If your primary focus is achieving the highest material purity: CVD is an ideal method, as the purity of the precursor gases translates directly to a high-purity solid film.
- If your primary focus is cost-efficiency for simple, flat surfaces: A physical deposition method (PVD) might be a more practical and economical alternative.
- If your substrate is temperature-sensitive: You must explore specialized low-temperature CVD variants (like Plasma-Enhanced CVD) or choose a different deposition technology altogether.
By mastering the interplay of gas, heat, and chemistry, you gain precise control over creating materials at the atomic level.
Summary Table:
| CVD Aspect | Key Detail |
|---|---|
| Process | Chemical reaction of precursor gases on a heated substrate |
| Key Advantage | Excellent conformal coverage on complex 3D shapes |
| Primary Benefit | Creates exceptionally pure, high-performance materials |
| Main Limitation | Typically requires high temperatures and a vacuum environment |
Need to create ultra-pure, high-performance coatings for your lab's research or production? The CVD process requires precise control and reliable equipment. KINTEK specializes in high-quality lab equipment and consumables, serving the exacting needs of laboratories. Our experts can help you select the right tools to master material creation at the atomic level. Contact us today via our form to discuss your specific application and how we can support your success with CVD technology.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Laboratory CVD Boron Doped Diamond Materials
People Also Ask
- What temperature does CVD graphene grow? Mastering the Critical Thermal Window
- What is the temperature of a CVD furnace? From 200°C to 1600°C for Precise Film Deposition
- What is the difference between CVD and sputter coating? Choose the Right Thin-Film Deposition Method
- What are the general steps involved in the HTCVD process? Mastering High-Temperature Film Deposition
- Is CVD a chemical process used to produce high-performance materials? Engineer Advanced Materials from the Atom Up
- What is atmospheric pressure chemical vapor deposition? A Fast, Cost-Effective Thin-Film Solution
- What are the steps in chemical vapor deposition? A Guide to Controlled Thin-Film Synthesis
- Which one is better, HPHT or CVD? Choosing the Right Lab-Grown Diamond for Your Priorities



















