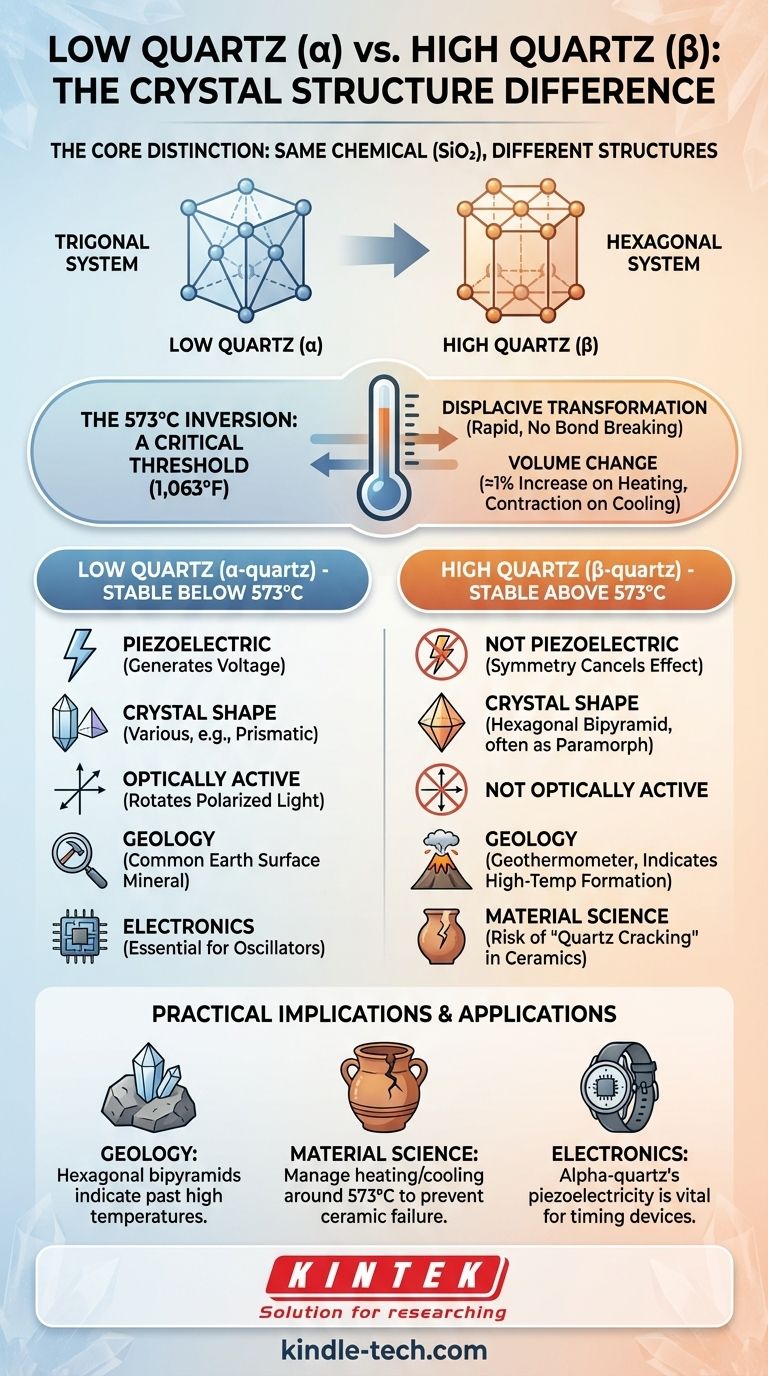
The fundamental difference is that low quartz and high quartz are two different crystal structures of the same chemical compound, silicon dioxide (SiO₂). Low quartz, or alpha-quartz (α-quartz), is the stable form at temperatures below 573°C (1,063°F). High quartz, or beta-quartz (β-quartz), is the stable form at temperatures above this point. This temperature-driven structural change is the source of all their differing properties.
The distinction between low (alpha) and high (beta) quartz is not a difference in chemical composition but in crystal symmetry. This polymorphic transformation at 573°C dictates the material's physical properties, determining its use in everything from geology to electronics.

The Core Distinction: Crystal Structure and Symmetry
The atomic arrangement within a crystal defines its properties. While both forms are made of SiO₄ tetrahedra, how those tetrahedra are linked and oriented changes with temperature.
### Low Quartz (α-quartz): The Everyday Form
Low quartz, or alpha-quartz, is the form of quartz stable under the conditions of the Earth's surface. Virtually all natural quartz you encounter is alpha-quartz.
Its crystal structure belongs to the trigonal crystal system. This lower-symmetry arrangement is what gives rise to some of its most famous properties.
### High Quartz (β-quartz): The High-Temperature Form
High quartz, or beta-quartz, only forms and remains stable at high temperatures, specifically between 573°C and 870°C.
Its structure belongs to the hexagonal crystal system. It has a higher degree of symmetry than alpha-quartz because the atoms have more thermal energy and vibrate into a less constrained arrangement.
The 573°C Inversion: A Critical Threshold
The change from alpha to beta quartz is a rapid, reversible, and non-destructive process known as a displacive transformation. No chemical bonds are broken; the atoms merely shift their positions slightly.
### The Transition Point
At 1 atmosphere of pressure, this inversion happens precisely at 573°C. As pressure increases, the transition temperature also increases slightly.
This transition is instantaneous. When beta-quartz cools below 573°C, it immediately inverts back to alpha-quartz.
### The Volume Change
The structural shift is accompanied by a sudden, small increase in volume of about 1% when going from alpha to beta.
Conversely, there is a sudden contraction upon cooling. This change can induce stress and micro-fracturing in rocks or ceramics that contain quartz.
Key Differences in Physical Properties
The change in crystal symmetry has profound effects on the material's physical behavior. This is the "why it matters" behind the distinction.
### Piezoelectricity
Alpha-quartz is piezoelectric, meaning it generates an electrical voltage when mechanical stress is applied. This property is a direct result of its lower-symmetry trigonal structure. This makes it essential for electronics like watches and radio oscillators.
Beta-quartz is not piezoelectric. Its higher hexagonal symmetry cancels out this effect.
### Crystal Shape (Morphology)
Beta-quartz typically crystallizes as a hexagonal bipyramid (two six-sided pyramids joined at their base).
When this beta-quartz crystal cools and inverts to alpha-quartz, it keeps the original hexagonal shape. Geologists call this a paramorph. Finding quartz with this shape is a key indicator that the rock it's in formed at a temperature above 573°C.
### Optical Properties
Alpha-quartz is optically active, meaning it can rotate the plane of polarized light. This is also a function of its lower-symmetry, "twisted" trigonal structure. Beta-quartz does not have this property.
Practical Implications and Applications
Understanding this transformation is not just an academic exercise; it has critical real-world consequences.
### In Geology
The quartz inversion is a powerful geothermometer. If a geologist finds quartz crystals with a beta-quartz shape (hexagonal bipyramids), they know with certainty that the host rock must have formed or been heated above 573°C.
### In Material Science and Ceramics
The sudden volume change at 573°C is a major concern when firing ceramics that contain quartz sand or clay. Heating or cooling too quickly through this temperature can cause the material to crack, a phenomenon known as "quartz cracking" or dunting.
### In Electronics
The piezoelectric property of alpha-quartz is the foundation of the modern electronics industry. For a quartz crystal oscillator to function, it must be alpha-quartz and must always be operated at temperatures well below the 573°C inversion point to maintain its critical structure.
Making the Right Choice for Your Goal
Your reason for asking about the difference determines which properties are most important to you.
- If your primary focus is mineral identification: Look for crystal shape. A hexagonal bipyramid indicates the crystal originally formed as high-temperature beta-quartz, even though it is now alpha-quartz.
- If your primary focus is engineering electronic devices: You must use alpha-quartz for its piezoelectric properties and ensure its operating environment never approaches the 573°C transition temperature.
- If your primary focus is working with ceramics or high-temperature materials: You must carefully manage the heating and cooling rates around 573°C to avoid structural failure from the rapid volume change.
Ultimately, understanding this temperature-driven structural shift is the key to predicting and harnessing the behavior of quartz across science and industry.
Summary Table:
| Property | Low Quartz (α-quartz) | High Quartz (β-quartz) |
|---|---|---|
| Stable Temperature | Below 573°C (1,063°F) | Above 573°C to 870°C |
| Crystal System | Trigonal | Hexagonal |
| Piezoelectric | Yes | No |
| Optical Activity | Optically active | Not optically active |
| Common Form | All natural quartz at Earth's surface | Forms only at high temperatures |
Need Precision Lab Equipment for Your Quartz Research?
Understanding the nuances of quartz behavior is crucial for success in geology, materials science, and electronics. Whether you're studying phase transitions, developing new ceramics, or manufacturing piezoelectric devices, having the right equipment is essential.
KINTEK specializes in providing high-quality lab equipment and consumables tailored to your specific research needs. We can help you with:
- Precision Heating Systems: For controlled studies of the 573°C quartz inversion point
- Material Analysis Tools: To characterize crystal structure and properties
- Ceramic Processing Equipment: Designed to manage the quartz transition safely
Let us help you achieve more accurate and reliable results. Contact our experts today to discuss how our solutions can support your work with quartz and other advanced materials.
Visual Guide
Related Products
- High Temperature Resistant Optical Quartz Glass Sheet
- Laboratory CVD Boron Doped Diamond Materials
- 100L Chilling Circulator Cooling Water Circulator for Low Temperature Constant Temperature Reaction Bath Water Bath Cooling
- 30L Chiller Water Bath Cooling Circulator Low Temperature Constant Temperature Reaction Bath
- 50L Chiller Water Bath Cooling Circulator Low Temperature Constant Temperature Reaction Bath
People Also Ask
- What are the advantages and disadvantages of bio-oil? A Guide to This Renewable Fuel
- What is the importance of determining the melting point of a substance? Identify Compounds & Assess Purity
- What is the process of sputtering in a vacuum? A Guide to High-Purity Thin Film Deposition
- What are the advantages of using sinter? Achieve High-Strength, Complex Parts with Minimal Waste
- What are the common designs of ultra-low temperature freezers? Upright vs. Chest Models for Your Lab
- Why is a precision temperature control system critical for UFG 304L stainless steel irradiation experiments?
- How do diameter and bed height affect aluminum powder fluidization? Master Design Ratios for Uniform Oxidation
- What is the advantage of magnetron sputtering? High-Quality, Dense Thin Films at High Deposition Rates







