At its core, FTIR is not a different technique from IR, but rather a superior method for performing it. The true distinction is between Fourier-Transform Infrared (FTIR) spectroscopy and the older, slower method of dispersive IR spectroscopy. While both use infrared light to analyze a sample's molecular structure, FTIR collects all spectral data simultaneously, whereas dispersive IR scans through each wavelength one by one.
The essential difference lies in the instrumentation and data acquisition. An FTIR spectrometer uses an interferometer to measure all frequencies at once, offering immense advantages in speed, sensitivity, and accuracy over traditional dispersive instruments that use a monochromator to measure frequencies sequentially.

What is Infrared (IR) Spectroscopy?
The Fundamental Principle
Infrared (IR) spectroscopy is a technique that probes the vibrations of molecules. When a molecule is exposed to infrared radiation, its chemical bonds will absorb energy and vibrate by stretching, bending, or rotating.
Different types of bonds (like C-H, O-H, or C=O) absorb light at different, specific frequencies. A spectrometer measures which frequencies of light are absorbed by the sample.
The "Fingerprint" Spectrum
The resulting plot of absorbance versus frequency (or wavenumber) is an IR spectrum. This spectrum acts as a unique "molecular fingerprint," allowing chemists to identify the functional groups present in a sample and ultimately determine its chemical identity.
The Core Difference: How the Spectrum is Measured
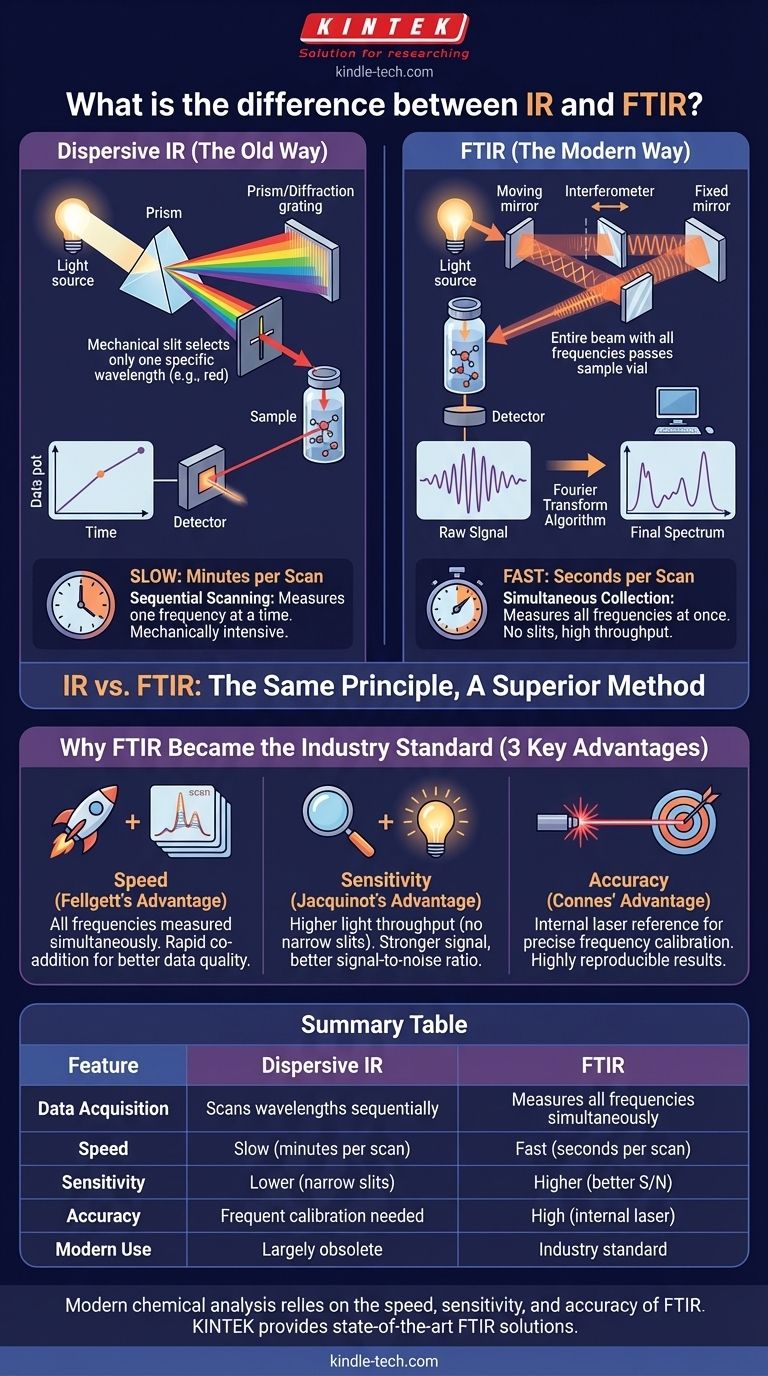
The terms "IR" and "FTIR" both refer to the same fundamental principle, but they describe two vastly different generations of instruments for collecting the data.
The Old Way: Dispersive IR Spectroscopy
Historically, an "IR spectrometer" was a dispersive instrument. It used a component like a prism or a diffraction grating to physically separate the infrared light into its constituent frequencies, much like a prism separates white light into a rainbow.
A narrow mechanical slit would then select one specific frequency at a time to pass through the sample to a detector. To generate a full spectrum, the grating would have to be slowly rotated to scan through the entire frequency range, step-by-step. This process was often slow, taking several minutes, and mechanically intensive.
The Modern Way: Fourier-Transform IR (FTIR) Spectroscopy
An FTIR spectrometer replaces the slow dispersive components (grating and slit) with an optical device called an interferometer, most commonly a Michelson interferometer.
Instead of scanning one frequency at a time, the interferometer allows a broad range of IR frequencies to pass through the sample to the detector simultaneously. The raw signal produced, called an interferogram, is a complex plot of light intensity versus the position of a moving mirror inside the interferometer.
The Role of the Fourier Transform
This raw interferogram is not human-readable as a spectrum. A computer then applies a mathematical operation called a Fourier Transform to this signal. This algorithm instantly converts the complex time-domain signal (the interferogram) into the familiar frequency-domain signal (the absorbance spectrum).
Why FTIR Became the Industry Standard
FTIR did not just incrementally improve upon dispersive IR; it completely revolutionized the technique by overcoming its fundamental limitations. This is due to three key advantages.
The Speed Advantage (Fellgett's Advantage)
Because all frequencies are measured at the same time (the multiplex principle), a complete scan can be completed in about one second. A dispersive instrument would take that long to measure just one data point. This speed allows for the rapid co-addition of multiple scans, dramatically improving data quality.
The Sensitivity Advantage (Jacquinot's Advantage)
Dispersive instruments require narrow slits to achieve good spectral resolution, which severely limits the amount of light (energy) that reaches the detector. FTIR instruments have no such slits, allowing much higher light throughput. This results in a much stronger signal and a far better signal-to-noise ratio, making FTIR ideal for analyzing weak or very small samples.
The Accuracy Advantage (Connes' Advantage)
FTIR instruments include an internal helium-neon (HeNe) laser as a constant reference for the optical path. This ensures the frequency axis (x-axis) of the spectrum is extremely accurate and perfectly reproducible from scan to scan and instrument to instrument. Dispersive instruments suffer from lower accuracy and require frequent recalibration.
Understanding the Trade-offs
The Obsolescence of Dispersive IR
For nearly all modern applications in research, quality control, and forensics, FTIR is the only method used. The advantages in speed, sensitivity, and accuracy are so overwhelming that dispersive IR instruments are now considered obsolete for general-purpose analysis.
The Complexity of FTIR
The primary "trade-off" is that FTIR is more complex. It relies on a high-precision optical device (the interferometer) and requires a computer with software to perform the Fourier Transform. However, decades of development have made modern FTIR spectrometers reliable, affordable, and easy-to-use "black box" systems.
Making the Right Choice for Your Goal
- If your primary focus is modern chemical analysis: You will be using and discussing FTIR. It is the dominant, superior technology, and for most chemists today, "IR spectroscopy" and "FTIR spectroscopy" are used interchangeably to refer to the modern technique.
- If your primary focus is reading older scientific literature (pre-1980s): Be aware that a spectrum labeled "IR" was almost certainly collected on a slower, less accurate dispersive instrument.
- If your primary focus is distinguishing the general concept from the instrument: Use "IR spectroscopy" to describe the broad scientific field and "FTIR spectrometer" to describe the modern instrument that performs the measurement.
Understanding this distinction clarifies why modern chemical identification relies on the speed, sensitivity, and precision delivered by Fourier-Transform technology.
Summary Table:
| Feature | Dispersive IR | FTIR |
|---|---|---|
| Data Acquisition | Scans wavelengths sequentially | Measures all frequencies simultaneously |
| Speed | Slow (minutes per scan) | Fast (seconds per scan) |
| Sensitivity | Lower (due to narrow slits) | Higher (better signal-to-noise ratio) |
| Accuracy | Requires frequent calibration | High (internal laser reference) |
| Modern Use | Largely obsolete | Industry standard |
Ready to enhance your laboratory's analytical capabilities?
At KINTEK, we specialize in providing state-of-the-art FTIR spectrometers and lab equipment that deliver the speed, sensitivity, and accuracy your research demands. Whether you're in quality control, forensics, or materials science, our solutions are designed to streamline your workflow and provide reliable results.
Contact us today to discuss your specific needs and discover how KINTEK can support your laboratory's success. Get in touch now!
Visual Guide
Related Products
- Lab Infrared Press Mold
- Three-dimensional electromagnetic sieving instrument
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Infrared High Resistance Single Crystal Silicon Lens
- Infrared Thermal Imaging Temperature Measurement Double-Sided Coated Germanium Ge Lens
People Also Ask
- What are sintered products used for? From Gears to Medical Implants, Discover Their Versatility
- What is sintering glass? A Low-Temperature Process for Complex Glass Parts
- What are the properties of isotropic graphite? A Guide to Its Uniform Strength & Thermal Performance
- How many types of physical vapor deposition are there? The 4 Main PVD Processes Explained
- What is the purpose of vacuum filtration equipment in gallium leaching? Achieve Rapid Solid-Liquid Separation Efficiency
- What does the sputtering yield depend on? Master the Physics for Optimal Thin Film Deposition
- What materials are needed for a FTIR? Essential Guide to Sample Prep and Optics
- What are the guidelines for sintering design? A Systematic Approach to Material Density and Strength


















