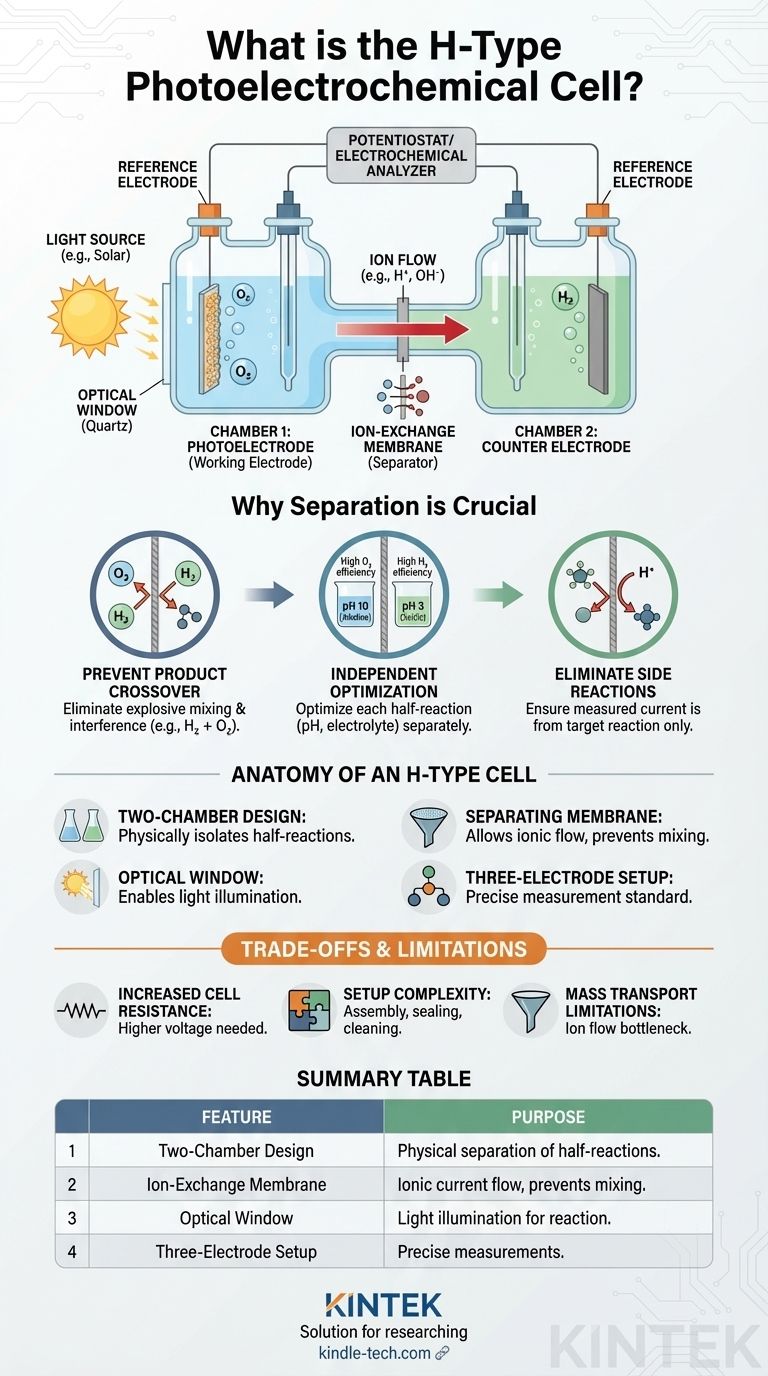
At its core, an H-type photoelectrochemical cell (PEC) is a specialized two-compartment electrochemical cell designed for studying light-driven chemical reactions. Its signature 'H' shape consists of two separate chambers connected by a bridge, which houses a membrane to keep the solutions in each chamber from mixing while still allowing ions to pass through. This design also features a transparent optical window in one chamber, allowing a light source to illuminate the working electrode.
The fundamental purpose of the H-type cell is to physically separate the two half-reactions of a photoelectrochemical process. This separation allows researchers to independently control and analyze the oxidation and reduction reactions, which is impossible in a standard single-compartment cell.

The Anatomy of an H-Type Cell
An H-type cell is a purpose-built tool for precise electrochemical analysis. Its design directly addresses the common challenges encountered when studying complex reactions like water splitting or CO2 reduction.
The Two-Compartment Design
The cell is constructed from two vertical glass chambers joined by a horizontal tube, forming a distinct 'H' shape. One chamber contains the photoelectrode (the working electrode) in an electrolyte, while the other contains the counter electrode in a separate electrolyte.
The Separating Membrane
The bridge connecting the two chambers holds a separator, typically an ion-exchange membrane (like Nafion) or a porous glass frit. This membrane is the key to the cell's function: it allows ions to flow between the chambers to complete the electrical circuit but prevents the bulk mixing of the two electrolytes.
The Optical Window
One of the chambers is built with an optical window made of a material like quartz, which is transparent to UV and visible light. This allows a light source to be precisely aimed at the photoelectrode, initiating the light-dependent reaction being studied.
Versatile Electrode Configuration
The design supports a full three-electrode setup, which is the standard for accurate electrochemical measurements. The working electrode (the material being tested) and reference electrode are placed in one chamber, while the counter electrode is placed in the other. This isolates the reactions at the working and counter electrodes.
Why Separation is Crucial in Photoelectrochemistry
The main reason to use an H-type cell is to prevent the products and reactants from one half-reaction from interfering with the other. This separation is critical for obtaining accurate and meaningful data.
Preventing Product Crossover
Consider the example of water splitting. At the photoanode, light helps generate oxygen (O₂). At the cathode, hydrogen (H₂) is produced. If these gases were allowed to mix, as they would in a single-compartment cell, they could re-react or create an explosive mixture, ruining the measurement and posing a safety risk.
Independent Optimization of Half-Reactions
The optimal conditions for different reactions can vary dramatically. For instance, the oxygen evolution reaction often works best in an alkaline (high pH) solution, while the CO₂ reduction reaction is more efficient in a neutral or slightly acidic solution. The H-type cell allows you to maintain a different pH and electrolyte composition in each chamber, maximizing the efficiency of both half-reactions simultaneously.
Eliminating Unwanted Side Reactions
By isolating the two halves of the cell, you ensure that the product generated at one electrode doesn't migrate to the other electrode and undergo an unwanted reaction. This isolation guarantees that the current you measure is a direct result of the specific reaction you intend to study.
Understanding the Trade-offs and Limitations
While powerful, the H-type cell is not without its drawbacks. Its specialized design introduces complexities that researchers must manage.
Increased Cell Resistance
The membrane separating the two compartments adds significant ionic resistance to the system. This resistance means a higher voltage (overpotential) is required to drive the reaction, which can lower the overall energy efficiency of the process.
Complexity in Setup
Compared to a simple beaker cell, an H-type cell is more complex to assemble, clean, and seal. Ensuring the cell is leak-proof is critical, as any contamination between the two chambers can invalidate the results of a long experiment.
Mass Transport Limitations
The rate at which ions can travel through the membrane can become a bottleneck, especially in experiments designed to run at high currents. If ions cannot move fast enough, it can limit the overall speed of the reaction you are trying to measure.
Choosing the Right Cell for Your Experiment
The decision to use an H-type cell depends entirely on the goal of your research.
- If your primary focus is fundamental research on a specific half-reaction: The H-type cell is the ideal choice for isolating and studying either oxidation or reduction without interference.
- If your primary focus is developing a complete device (e.g., for water splitting or CO₂ conversion): The H-type cell is essential for testing and optimizing the anolyte and catholyte independently before integration.
- If your primary focus is rapid screening of new materials: A simpler, single-compartment cell is often more practical for quickly assessing the basic photoactivity of many materials, as it avoids the setup complexity of the H-type cell.
Selecting the correct experimental setup is the first step toward designing a precise and meaningful photoelectrochemical experiment.
Summary Table:
| Feature | Purpose |
|---|---|
| Two-Chamber Design | Physically separates the oxidation and reduction half-reactions. |
| Ion-Exchange Membrane | Prevents reactant/product mixing while allowing ionic current flow. |
| Optical Window (Quartz) | Allows light to illuminate the photoelectrode for reaction initiation. |
| Three-Electrode Setup | Enables precise electrochemical measurements with isolated electrodes. |
Ready to conduct precise photoelectrochemical research?
An H-type cell is essential for accurately studying complex reactions like water splitting and CO2 reduction. KINTEK specializes in providing high-quality laboratory equipment, including specialized electrochemical cells and consumables, to support your research needs.
Contact us today to discuss how our solutions can enhance your lab's capabilities and help you achieve reliable, interference-free results. Get in touch via our contact form and let our experts assist you.
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Thin-Layer Spectral Electrolysis Electrochemical Cell
People Also Ask
- What is the structure of an H-type exchangeable membrane electrolytic cell? A Guide to Precise Electrochemical Separation
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- What are the key features of a double-layer water-bath electrolytic cell? Achieve Precise Temperature Control for Your Experiments
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup



















