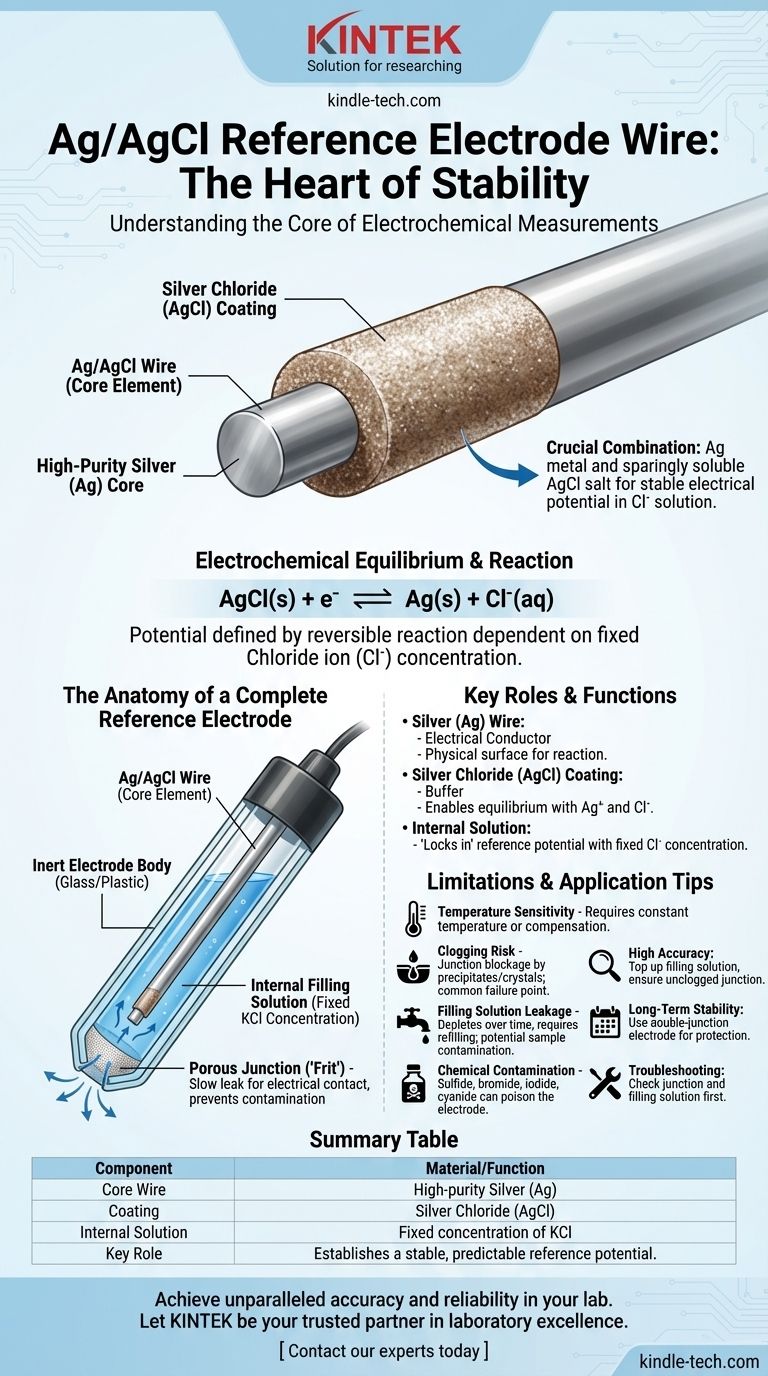
The wire at the core of a silver/silver chloride (Ag/AgCl) reference electrode is a high-purity silver (Ag) wire that has been coated with a layer of silver chloride (AgCl). This specific combination of a metal and its sparingly soluble salt is what allows the electrode to maintain a stable, predictable electrical potential when immersed in a chloride-containing solution.
The stability of an Ag/AgCl electrode doesn't come from the silver wire alone. It arises from the electrochemical equilibrium established between the metallic silver, its silver chloride coating, and the constant concentration of chloride ions in the solution filling the electrode.

How the Ag/AgCl Wire Creates a Stable Reference
To understand why this specific wire is used, we must look at it as one part of a precise electrochemical system. The potential of the electrode is not arbitrary; it is governed by a specific, reversible chemical reaction.
The Role of the Silver (Ag) Wire
The pure silver wire serves as the solid, metallic phase in the electrode. It is an excellent electrical conductor that provides the physical surface where the crucial electrochemical reaction takes place.
The Critical Silver Chloride (AgCl) Coating
The layer of silver chloride is the most critical component. AgCl is a salt that is only sparingly soluble in water. This coating acts as a buffer, ensuring the silver wire is always in equilibrium with a source of both silver ions (Ag⁺) and chloride ions (Cl⁻).
The Defining Electrochemical Reaction
The stable potential of the electrode is defined by the following reversible reaction:
AgCl(s) + e⁻ ⇌ Ag(s) + Cl⁻(aq)
The potential of this reaction depends directly on the concentration of chloride ions (Cl⁻) in the solution. Because the electrode is filled with a solution of known and fixed chloride concentration, its potential remains stable and predictable.
Anatomy of a Complete Reference Electrode
The Ag/AgCl wire does not function in isolation. It is housed within a carefully designed body that ensures its potential remains constant, regardless of the sample solution it is measuring.
The Internal Filling Solution
The electrode body is filled with an electrolyte solution containing a fixed concentration of chloride ions, most commonly potassium chloride (KCl). This solution ensures the chloride ion concentration in the reaction above remains constant, which "locks in" the electrode's reference potential.
The Porous Junction (or 'Frit')
A porous plug, often made of ceramic or Teflon, is located at the tip of the electrode. Its job is to allow electrical contact (ion flow) between the internal filling solution and the external sample solution. It is designed to leak very slowly, preventing contamination of the reference element.
The Electrode Body
The body itself is typically made of inert glass or plastic tubing. It serves to house the Ag/AgCl wire and the internal filling solution, protecting them from the outside environment.
Understanding the Trade-offs and Limitations
While robust, Ag/AgCl electrodes are not without their operational constraints. Understanding these is key to achieving accurate measurements.
Temperature Sensitivity
The potential of an Ag/AgCl electrode is dependent on temperature. For highly precise work, measurements must be made at a constant, known temperature, or the temperature variation must be accounted for.
Risk of Clogging
The porous junction is the most common point of failure. If it becomes clogged by precipitates from the sample solution or by dried KCl crystals, the electrical circuit is broken, and the electrode will cease to function correctly.
Filling Solution Leakage
The junction must leak slowly to maintain ionic contact. This means the filling solution will be depleted over time and must be refilled. This leakage can also introduce small amounts of chloride and potassium ions into your sample, which may be a concern in certain sensitive analyses.
Chemical Contamination
The reference potential can become unstable if the electrode is exposed to substances that react with silver. Sulfide, bromide, iodide, and cyanide ions are particularly problematic and can "poison" the electrode surface.
How to Apply This to Your Experiment
When working with Ag/AgCl electrodes, your primary goal dictates how you should use and maintain them.
- If your primary focus is high-accuracy measurements: Always check that the filling solution is topped up and free of air bubbles or crystals, and ensure the porous junction is unclogged before each use.
- If your primary focus is long-term stability (e.g., in a monitoring setup): Use a double-junction electrode to protect the inner Ag/AgCl element from potential contaminants in your sample solution.
- If your primary focus is troubleshooting a faulty reading: Suspect a clogged junction or depleted filling solution first, as these are the most common and easily fixable failure modes for a reference electrode.
Understanding that the Ag/AgCl wire is the heart of a complete electrochemical system is the key to achieving reliable and repeatable measurements.
Summary Table:
| Component | Material/Function |
|---|---|
| Core Wire | High-purity Silver (Ag) |
| Coating | Silver Chloride (AgCl) |
| Internal Solution | Fixed concentration of KCl |
| Key Role | Establishes a stable, predictable reference potential |
Achieve unparalleled accuracy and reliability in your lab.
The silver/silver chloride (Ag/AgCl) reference electrode is fundamental for precise electrochemical measurements. At KINTEK, we understand that your research demands the highest level of consistency and performance from your lab equipment.
We provide high-quality reference electrodes and consumables designed for durability and accuracy, ensuring your experiments are never compromised. Whether you are conducting sensitive pH analysis, potentiometric titrations, or long-term monitoring, the right equipment is critical.
Let KINTEK be your trusted partner in laboratory excellence. Contact our experts today to find the perfect electrochemical solutions for your specific application and ensure your measurements are always on point.
Visual Guide
Related Products
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Disc Electrode
- Copper Sulfate Reference Electrode for Laboratory Use
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
- Platinum Auxiliary Electrode for Laboratory Use
People Also Ask
- How should a platinum wire/rod electrode be cleaned before use? A Guide to Reliable Electrochemical Data
- What is the RRDE in electrochemistry? Unlock Detailed Reaction Pathways with Dual-Electrode Analysis
- What is a common application for the platinum wire/rod electrode? The Essential Guide to Counter Electrodes
- What is the application of RRDE? Unlock Quantitative Catalyst and Reaction Insights
- What is the difference between RDE and RRDE? Unlock Advanced Electrochemical Reaction Analysis















