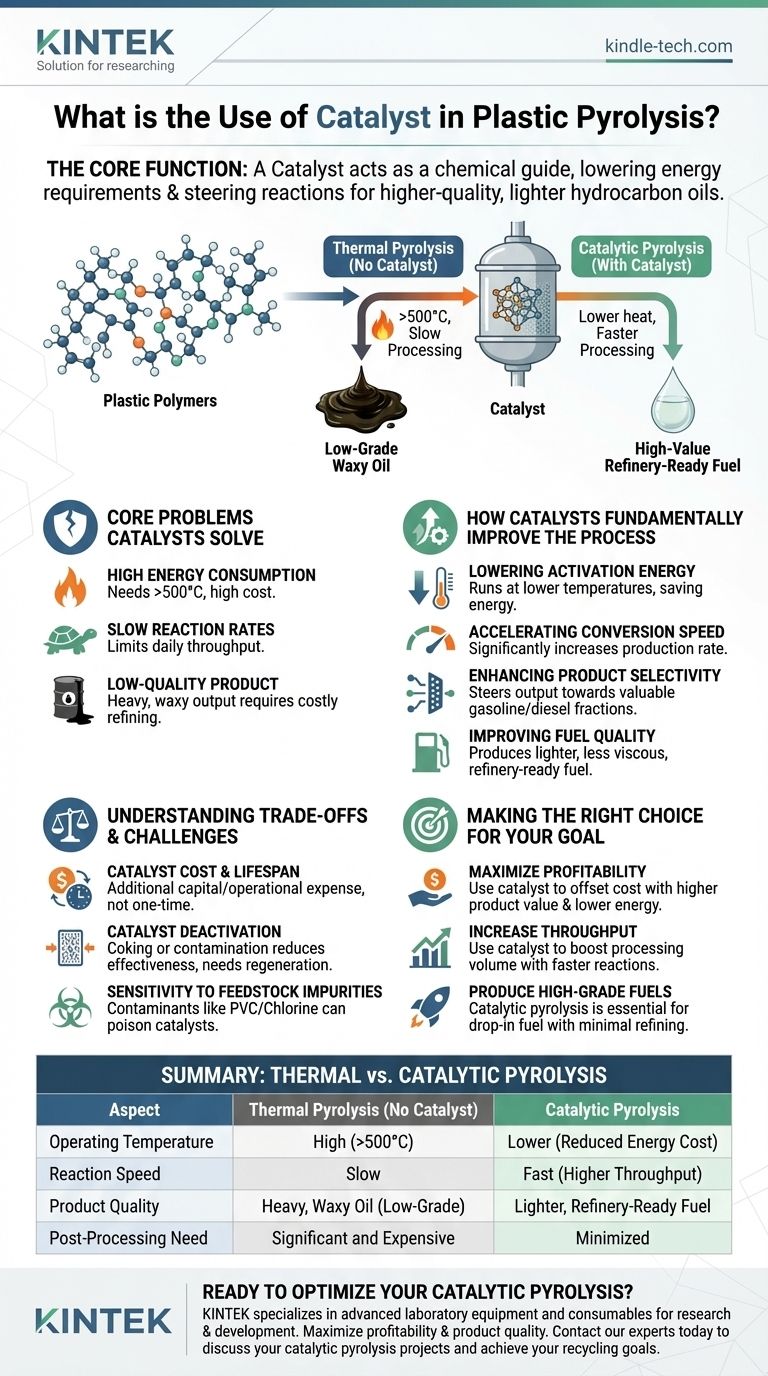
In plastic pyrolysis, a catalyst is a substance that makes the process more efficient and produces a higher-quality final product. It acts as a chemical guide, lowering the energy required to break down plastic polymers and steering the reactions to create more valuable, lighter hydrocarbon oils instead of heavy, waxy tars.
The fundamental purpose of a catalyst in plastic pyrolysis is to solve the core economic and technical challenges of the process. It transforms a slow, energy-intensive operation that produces low-grade oil into a faster, more energy-efficient process that yields a more valuable, refinery-ready fuel.

The Core Problems Catalysts Solve in Pyrolysis
To understand the value of a catalyst, we must first understand the limitations of basic thermal pyrolysis (using heat alone). This process faces significant hurdles that hinder its commercial viability.
High Energy Consumption
Without a catalyst, breaking the strong chemical bonds in plastics like polypropylene and polyethylene requires extremely high temperatures, often exceeding 500°C. Maintaining these temperatures consumes a vast amount of energy, which represents a major operational cost.
Slow Reaction Rates
The thermal decomposition of plastics is a relatively slow process. This slow conversion rate limits the amount of waste plastic a facility can process per day, directly impacting its overall throughput and profitability.
Low-Quality Product
Perhaps the most significant challenge is the output. Thermal pyrolysis often produces a heavy, waxy, and inconsistent mixture of hydrocarbons. This "pyrolysis oil" requires significant and expensive secondary refining (hydrotreating or upgrading) before it can be used as a fuel.
How Catalysts Fundamentally Improve the Process
Introducing a catalyst into the pyrolysis reactor fundamentally changes the chemistry of the process, directly addressing the challenges of thermal-only methods.
Lowering the Activation Energy
A catalyst provides an alternative chemical pathway for the reaction to follow—one that requires less energy. This allows the pyrolysis process to run effectively at lower temperatures, significantly reducing energy consumption and operational costs.
Accelerating Conversion Speed
By providing a more efficient reaction pathway, a catalyst dramatically increases the rate at which plastic polymers are broken down into smaller molecules. This means more plastic can be converted into oil in a shorter amount of time, boosting the plant's overall capacity.
Enhancing Product Selectivity
This is the most critical function. Catalysts, such as zeolites, have specific structures that can selectively "crack" the long hydrocarbon chains into desired lengths. This allows operators to steer the chemical output towards the most valuable fractions, such as the molecules found in gasoline or diesel.
Improving Fuel Quality
As a direct result of enhanced selectivity, the final oil is of much higher quality. It is a lighter, less viscous liquid with a chemical composition closer to that of conventional fuels. This significantly reduces the need for costly post-processing and upgrading.
Understanding the Trade-offs and Challenges
While catalytic pyrolysis is a superior technology, it is essential to be aware of its operational complexities. An objective assessment requires acknowledging the associated challenges.
Catalyst Cost and Lifespan
Catalysts, particularly specialized ones, represent an additional capital and operational expense. They are not a one-time purchase.
Catalyst Deactivation
Over time, the catalyst's surface can become blocked by carbon deposits (a process known as "coking") or contaminated by impurities in the plastic waste. This deactivation reduces its effectiveness and requires periodic regeneration or complete replacement.
Sensitivity to Feedstock Impurities
The performance of a catalyst can be severely hindered by contaminants commonly found in post-consumer plastic waste. Chlorine from PVC plastic, for example, can permanently poison many types of catalysts, rendering them useless.
Making the Right Choice for Your Goal
The decision to use a catalyst—and which one—depends entirely on the technical and economic goals of the operation.
- If your primary focus is maximizing profitability: A catalyst is essential, as it lowers energy costs and increases the market value of your final product, offsetting its own expense.
- If your primary focus is increasing processing throughput: The accelerated reaction rates enabled by a catalyst are the most direct way to increase the volume of waste plastic your facility can handle.
- If your primary focus is producing high-grade fuels directly: Catalytic pyrolysis is the only viable path to creating a drop-in fuel that requires minimal secondary refining.
Ultimately, the catalyst is the key technology that elevates plastic pyrolysis from a crude decomposition method to a sophisticated chemical recycling process.
Summary Table:
| Aspect | Thermal Pyrolysis (No Catalyst) | Catalytic Pyrolysis |
|---|---|---|
| Operating Temperature | High (>500°C) | Lower (Reduced Energy Cost) |
| Reaction Speed | Slow | Fast (Higher Throughput) |
| Product Quality | Heavy, Waxy Oil (Low-Grade) | Lighter, Refinery-Ready Fuel |
| Post-Processing Need | Significant and Expensive | Minimized |
Ready to transform your plastic waste into high-value fuel efficiently? KINTEK specializes in advanced laboratory equipment and consumables for pyrolysis research and development. Our solutions help you optimize catalyst performance and process parameters to maximize profitability and product quality. Contact our experts today to discuss how we can support your catalytic pyrolysis projects and help you achieve your recycling goals.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Advanced Engineering Fine Ceramics Boron Nitride (BN) Ceramic Parts
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
People Also Ask
- Can you regenerate activated charcoal? The Truth About Reusing Spent Carbon Filters
- What is pyrolysis of lignocellulosic materials? Converting Biomass into Bio-oil, Bio-char, and Syngas
- What are the applications of rotary kiln? A Guide to Industrial Thermal Processing
- Which of the following process is an example of calcination? A Guide to Thermal Decomposition
- Can reactors be used for the pyrolysis of plastic waste? The Core Technology Explained
- What are the catalysts for fast pyrolysis? Overcoming Biomass Conversion Challenges
- What is the temperature of a rotating kiln? It Depends on Your Material and Process Goal
- What is the difference between regeneration and reactivation of activated carbon? Maximize Carbon Lifespan & Performance



















