In quantitative analysis, X-ray Fluorescence (XRF) is a technique used to determine the precise concentration or amount of specific elements within a sample. This is achieved by measuring the intensity of the characteristic X-rays emitted by the sample’s elements and comparing those intensities to measurements from a reference material with a known concentration.
While qualitative XRF answers what elements are present, quantitative XRF answers the critical question of how much of each element is there. This shift from identification to measurement is accomplished by benchmarking the sample’s X-ray signal against a known standard.
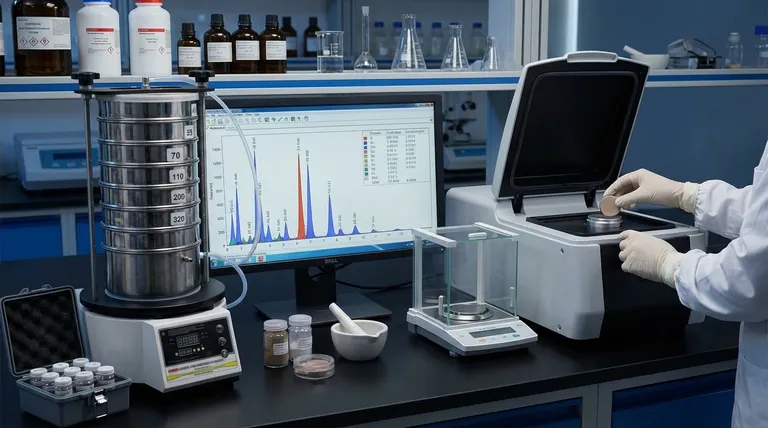
From Identification to Measurement: The Principle of Quantitative XRF
To use XRF effectively, it's crucial to understand the distinction between simply identifying an element and truly quantifying it.
Qualitative vs. Quantitative: The Two Goals of XRF
Qualitative analysis is the first step. Every element, when excited by X-rays, emits its own secondary X-rays at a unique and predictable energy level. This acts like a fingerprint, allowing the spectrometer to identify which elements are in the sample by noting the position (energy) of the peaks on a spectrum.
Quantitative analysis goes further. It measures the intensity (the height or area) of those energy peaks. The core principle is that a higher intensity corresponds to a higher concentration of that element in the sample.
The Role of the Standard
A raw intensity measurement is meaningless on its own. To convert it into a concentration value (like percentage or parts-per-million), it must be compared to a benchmark.
This benchmark is a calibration standard—a material that is physically similar to the sample but contains a precisely known concentration of the element you wish to measure. By comparing the intensity from the unknown sample to the intensity from the known standard, the instrument's software can calculate the concentration.
How the Spectrometer Gathers Data
The process is a straightforward chain of events. An X-ray source irradiates the sample, causing the atoms within it to become excited and emit their own fluorescent X-rays.
A detector collects these secondary X-rays and processes them into a spectrum. This spectrum is a graph that plots X-ray intensity against X-ray energy, providing the raw data for both qualitative and quantitative analysis.
Understanding the Trade-offs: The Challenge of Accuracy
While powerful, the accuracy of quantitative XRF is not automatic. It depends entirely on controlling key variables that can distort the results. Achieving precision requires a clear understanding of the potential pitfalls.
The Critical Impact of Sample Preparation
The physical state of your sample directly impacts the quality of the measurement. While XRF is often considered non-destructive, improper preparation is the most common source of error in quantitative analysis.
An uneven surface, inconsistent particle size in a powder, or variations in density can all scatter or absorb X-rays in an unpredictable way, leading to inaccurate intensity readings. Whether dealing with solid, powdered, or liquid samples, standardized preparation is non-negotiable for reliable results.
Matrix Effects: The Hidden Variable
The "matrix" is everything else in the sample besides the specific element you are measuring. These other elements are not passive; they can interfere with the measurement in two key ways.
They can absorb the X-rays emitted by your target element, reducing the signal that reaches the detector and causing an artificially low concentration reading. Conversely, they can enhance the signal through secondary fluorescence, leading to an artificially high reading. Correcting for these matrix effects is a primary function of good calibration and advanced analysis software.
Applying This to Your Analysis
Your approach to XRF should be dictated by your analytical goal. The level of rigor required changes significantly depending on whether you need a quick estimate or a certified, precise measurement.
- If your primary focus is rapid identification: Qualitative XRF is sufficient. You are primarily concerned with the energy position of the spectral peaks to know what is present.
- If your primary focus is precise concentration measurement: Quantitative XRF is necessary. This requires creating calibration curves from standards and implementing rigorous, repeatable sample preparation protocols.
- If you are analyzing materials with diverse compositions: You must be prepared to manage matrix effects. This may involve using more sophisticated correction models or developing multiple sets of calibration standards that closely match your different sample types.
Ultimately, understanding these core principles empowers you to move beyond simply generating data and toward producing truly accurate and defensible analytical results.
Summary Table:
| Aspect | Qualitative XRF | Quantitative XRF |
|---|---|---|
| Primary Goal | Identify which elements are present | Measure precise concentration of elements |
| Data Used | Energy position of spectral peaks | Intensity/height of spectral peaks |
| Key Requirement | None | Calibration standards with known concentrations |
| Accuracy Level | Element identification only | High precision with proper calibration |
| Sample Prep Importance | Low to moderate | Critical for reliable results |
Ready to achieve precise quantitative analysis in your lab?
KINTEK specializes in laboratory equipment and consumables, providing advanced XRF solutions that deliver accurate concentration measurements for your specific applications. Our expertise ensures you get reliable data with proper calibration and sample preparation guidance.
Contact us today to discuss how our XRF equipment can enhance your analytical capabilities and deliver the precise results your research demands!
Visual Guide
Related Products
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Custom PTFE Wafer Holders for Lab and Semiconductor Processing
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
- Lab Internal Rubber Mixer Rubber Kneader Machine for Mixing and Kneading
People Also Ask
- What steel is used for a hydraulic press? Choosing the Right Materials for High-Stress Performance
- Why is a high-tonnage hydraulic press required for UO2 ceramic pellets? Essential Force for Nuclear Fuel Density
- What makes a hydraulic press so powerful? Unlocking the Physics of Force Multiplication
- Why is a laboratory hydraulic press used for Li3V2(PO4)3 pellets? Optimize Solid-State Sintering for Li-ion Materials
- What is the purpose of using a laboratory hydraulic press in the preparation of unit cells? Enhance Data Integrity.
- What is the pressing process of ceramics? A Guide to Precise, High-Strength Manufacturing
- What are the disadvantages of KBr pellets? Avoid Moisture & Prep Errors in FTIR Analysis
- What are the limits of detection for XRF? Understanding Sensitivity for Accurate Analysis









