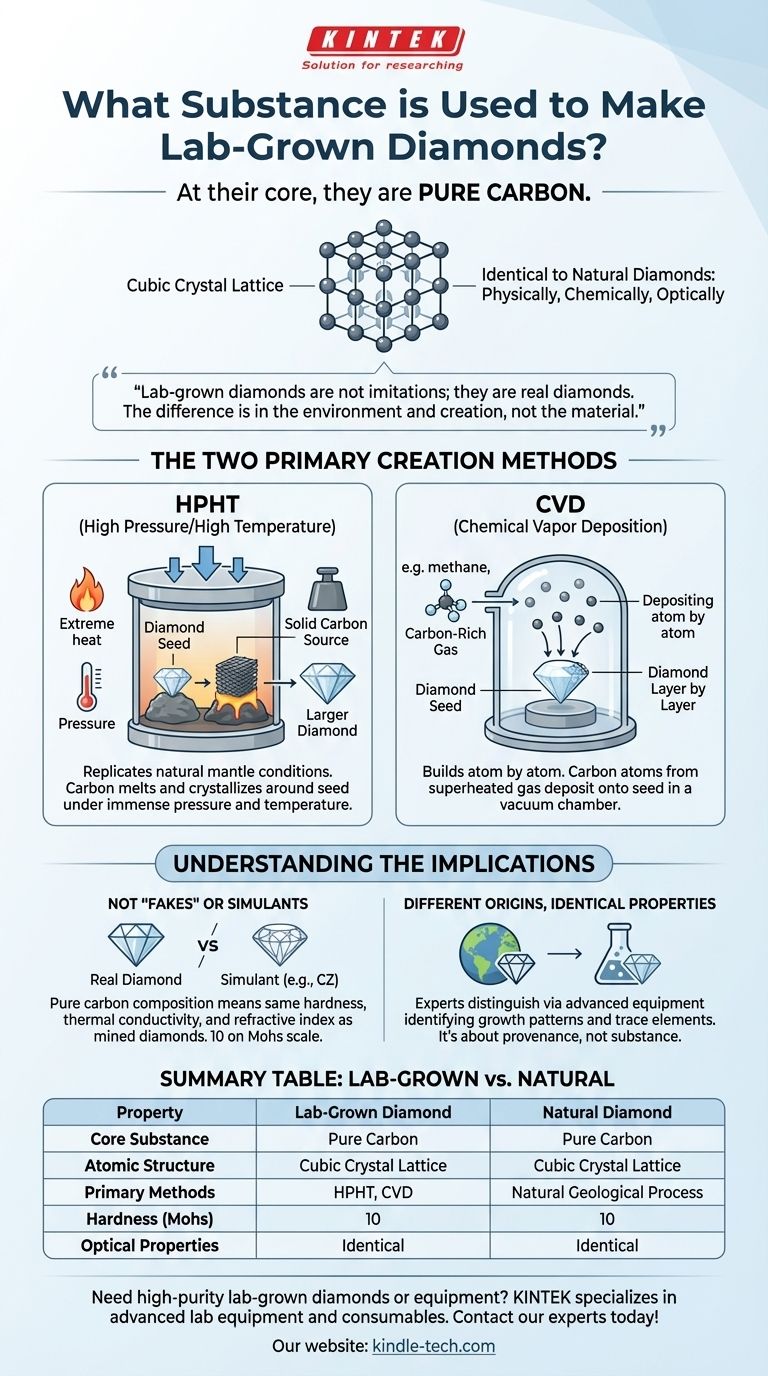
At their core, lab-grown diamonds are made of the exact same substance as natural diamonds: pure carbon. The carbon atoms are arranged in an identical crystal lattice structure, making them physically, chemically, and optically indistinguishable from diamonds formed in the Earth's mantle. The difference lies not in the material, but in the environment and method of their creation.
The essential takeaway is that lab-grown diamonds are not diamond imitations; they are real diamonds. The fundamental building block is carbon, which is transformed into a diamond crystal using one of two highly advanced manufacturing processes.
The Core Ingredient: Pure Carbon
A diamond's identity is defined by its atomic composition and structure. Lab-grown diamonds meet this definition perfectly.
An Identical Atomic Structure
Both natural and lab-created diamonds consist of carbon atoms bonded together in a rigid cubic crystal lattice. This specific arrangement is what gives a diamond its exceptional hardness and brilliance.
The Starting Material
The process begins with a source of carbon. In one method, this is often solid carbon like graphite. In another, it is a carbon-rich gas. This carbon is then subjected to specific conditions to encourage it to crystallize into a diamond.
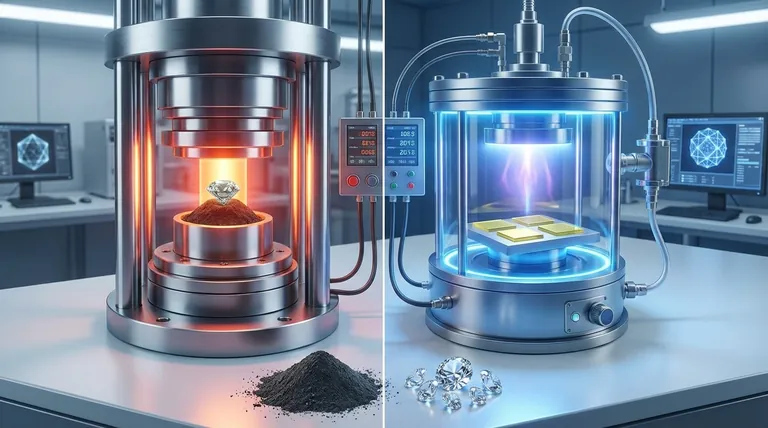
The Two Primary Creation Methods
Labs use two main techniques to create the precise environment needed for diamond formation. Both methods start with a tiny diamond "seed," which acts as a template for the new crystal to grow upon.
High Pressure/High Temperature (HPHT)
The HPHT method replicates the natural conditions deep within the Earth's mantle. A diamond seed is placed in a cell with solid carbon and subjected to immense pressure and extremely high temperatures.
This extreme environment causes the carbon to melt and crystallize around the seed, forming a new, larger diamond.
Chemical Vapor Deposition (CVD)
The CVD method takes a different approach, building the diamond atom by atom. A diamond seed is placed inside a sealed vacuum chamber filled with a carbon-rich gas, such as methane.
This gas is superheated, causing carbon atoms to break away and "deposit" onto the diamond seed. Over time, these atoms build up, growing the diamond layer by layer.
Understanding the Implications
Because lab-grown diamonds are made of pure carbon, they are not "fakes" or simulants like cubic zirconia. They are simply diamonds with a different origin story.
Real Diamonds, Different Origins
The final product from both HPHT and CVD processes is a real diamond. It possesses the same hardness (10 on the Mohs scale), thermal conductivity, and refractive index as a mined diamond.
Distinguishing Between Them
While visually identical to the naked eye, gemological experts can distinguish between lab-grown and natural diamonds. They use advanced equipment to identify minute differences in growth patterns and the presence of trace elements unique to each formation process.
Why This Matters
The distinction is about provenance, not substance. Choosing a lab-grown diamond is a decision based on factors like budget, environmental considerations, and ethical sourcing, not on the quality or authenticity of the material itself.
Making the Right Choice for Your Goal
Understanding the material composition helps clarify what a lab-grown diamond truly is.
- If your primary focus is jewelry: A lab-grown diamond is a real diamond in every physical and chemical sense, offering the same beauty and durability as a mined diamond.
- If your primary focus is authenticity: Understand that "lab-grown" signifies origin, not a difference in material. Both lab and mined diamonds are certified based on the same qualities of cut, color, clarity, and carat weight.
- If your primary focus is material science: Both HPHT and CVD produce structurally identical diamonds, but the specific growth process may introduce unique properties relevant for industrial or technological applications.
Ultimately, a diamond's identity is defined by its carbon structure, not its origin.
Summary Table:
| Property | Lab-Grown Diamond | Natural Diamond |
|---|---|---|
| Core Substance | Pure Carbon | Pure Carbon |
| Atomic Structure | Cubic Crystal Lattice | Cubic Crystal Lattice |
| Primary Methods | HPHT, CVD | Natural Geological Process |
| Hardness (Mohs Scale) | 10 | 10 |
| Optical Properties | Identical | Identical |
Need high-purity lab-grown diamonds or the equipment to create them? KINTEK specializes in advanced lab equipment and consumables for diamond synthesis and analysis. Whether you're in jewelry, research, or industrial manufacturing, our solutions ensure precision and quality. Contact our experts today to find the perfect diamond or equipment for your specific application!
Visual Guide
Related Products
- CVD Diamond for Thermal Management Applications
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- Custom CVD Diamond Coating for Lab Applications
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
People Also Ask
- What explains why carbon nanotubes make good catalysts? Unlock Their Unique Catalytic Power
- What is the method of sputtering? A Guide to High-Purity Thin Film Deposition
- Why is precise substrate heating essential for TiO2/Al-Zr bilayer thin films? Master AA-MOCVD Thermal Control
- What is deposition of thin film using sputtering methods? A Guide to High-Quality PVD Coating
- What are the disadvantages of chemical vapor deposition? High Costs, Safety Risks, and Material Limitations
- Why is the Chemical Vapor Deposition (CVD) process necessary for candle soot-templated silica? Enhancing Durability
- What is the deposition process? A Guide to Thin-Film Coating Techniques
- What is the function of the external reaction generator in a CVD aluminizing system? Achieve Precision Coating Control



















