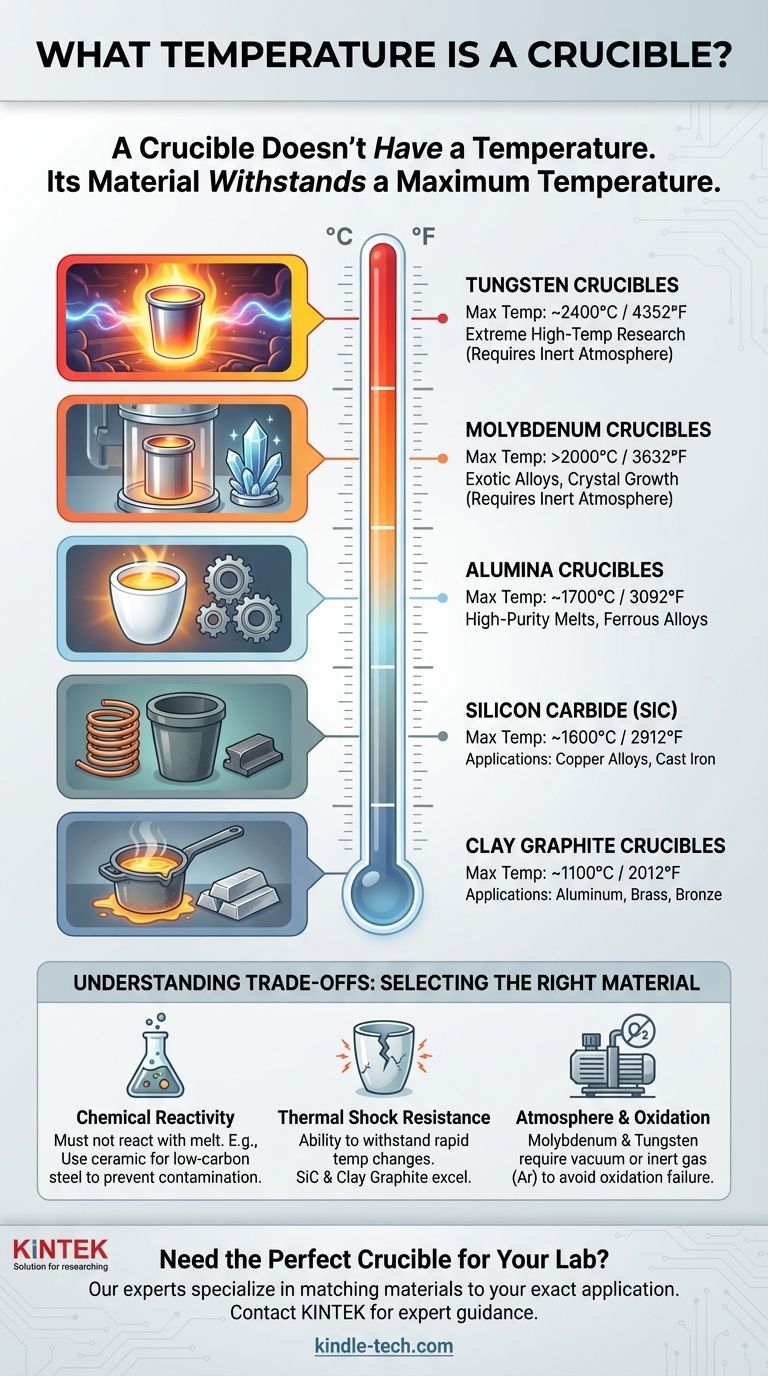
First, a critical distinction: A crucible does not have a temperature; it is a container designed to withstand a specific temperature. The maximum temperature a crucible can handle is determined entirely by the material it is made from, with common types ranging from around 1100°C (2012°F) for clay graphite up to and beyond 2000°C (3632°F) for specialized refractory metals like molybdenum.
The most important principle to understand is that there is no single "crucible temperature." The correct question is, "What material do I need for my target temperature?" Selecting a crucible is a process of matching its material properties to the specific demands of the substance you are heating.

Why Material Dictates Performance
The function of a crucible is to contain a substance while it is heated to a liquid state. Therefore, the crucible's melting point must be significantly higher than the melting point of the material inside it. Different crucible materials are engineered for different temperature ranges and chemical environments.
Clay Graphite Crucibles
This is one of the most common and cost-effective types of crucibles. They are primarily used for melting non-ferrous metals.
Their typical maximum service temperature is around 1100°C to 1200°C (2012°F to 2192°F), making them ideal for aluminum, brass, and bronze.
Silicon Carbide (SiC) Crucibles
Silicon carbide offers superior performance over clay graphite, with better thermal conductivity and greater strength at high temperatures.
These crucibles can handle more demanding applications, including copper alloys and even some cast iron, with operating temperatures up to 1600°C (2912°F).
Alumina Crucibles
Alumina (aluminum oxide) is a ceramic material known for its high purity and excellent chemical stability. This makes it suitable for applications where melt contamination is a concern.
They can withstand temperatures up to approximately 1700°C (3092°F) but can be more susceptible to thermal shock than graphite-based crucibles.
Molybdenum Crucibles
For extremely high-temperature applications, refractory metals are required. Molybdenum is a prime example.
As noted, the working temperature for a molybdenum crucible is generally above 2000°C (3632°F). These are used in specialized industrial or research settings, often for melting exotic alloys or growing crystals.
Tungsten Crucibles
Tungsten has the highest melting point of all metals, making it the choice for the most extreme temperature requirements.
Tungsten crucibles can be used at temperatures approaching 2400°C (4352°F), but like molybdenum, they are expensive and have specific operational needs.
Understanding the Trade-offs
Choosing a crucible involves more than just finding one that won't melt. You must consider the interplay between temperature, chemical compatibility, and operational constraints.
Chemical Reactivity
The crucible material should not react with the molten charge. For example, using a carbon-based (graphite) crucible to melt low-carbon steel will cause carbon to leach into the steel, changing its properties. In that case, a ceramic crucible like alumina or zirconia would be a better choice.
Thermal Shock Resistance
Thermal shock is the stress a material endures when its temperature changes rapidly, which can cause cracks. Materials like silicon carbide and clay graphite have excellent thermal shock resistance. Some high-purity ceramics, while stable at high temperatures, can be more brittle and must be heated and cooled more slowly.
Atmosphere and Oxidation
This is a critical factor for refractory metal crucibles. Materials like molybdenum and tungsten will rapidly oxidize and fail if heated in the presence of oxygen. They must be used in a vacuum or an inert gas atmosphere (like argon), which adds significant complexity and cost to the process.
Cost vs. Performance
There is a direct correlation between a crucible's performance and its cost. A clay graphite crucible might cost tens of dollars, while a large molybdenum or tungsten crucible can cost thousands. The goal is to select the most economical material that safely meets the technical requirements of your work.
Selecting the Right Crucible for Your Application
Your choice must be driven by your specific goal. Use this guide to make an informed decision.
- If your primary focus is melting common non-ferrous metals (like aluminum or brass): A clay graphite or silicon carbide crucible provides the best balance of performance and cost.
- If your primary focus is working with high-purity melts or ferrous alloys (like steel): You will need a chemically inert ceramic crucible, such as alumina or zirconia, to prevent contamination and handle the required heat.
- If your primary focus is extreme high-temperature research (above 2000°C): Refractory metal crucibles like molybdenum or tungsten are necessary, but you must operate them in a vacuum or inert atmosphere.
Matching the crucible's material to your specific temperature, chemical, and atmospheric requirements is the key to a safe and successful process.
Summary Table:
| Crucible Material | Typical Max Temperature (°C / °F) | Common Applications |
|---|---|---|
| Clay Graphite | 1100°C - 1200°C / 2012°F - 2192°F | Aluminum, Brass, Bronze |
| Silicon Carbide (SiC) | Up to 1600°C / 2912°F | Copper Alloys, Cast Iron |
| Alumina | Up to 1700°C / 3092°F | High-Purity Melts, Ferrous Alloys |
| Molybdenum | Above 2000°C / 3632°F | Exotic Alloys, Crystal Growth (Requires Inert Atmosphere) |
| Tungsten | Up to 2400°C / 4352°F | Extreme High-Temp Research (Requires Inert Atmosphere) |
Need the Perfect Crucible for Your Lab?
Choosing the right crucible is critical for the safety and success of your high-temperature processes. The experts at KINTEK specialize in matching laboratory equipment—from crucibles to full furnace systems—to your exact application requirements, whether you're working with common non-ferrous metals or extreme high-temperature research.
We provide:
- Expert Guidance: Get help selecting the ideal material (graphite, SiC, alumina, molybdenum, tungsten) based on your target temperature, chemical compatibility, and atmosphere.
- High-Quality Equipment: Access durable, reliable crucibles and related lab consumables designed for precise performance.
- Tailored Solutions: Find the perfect balance of performance, thermal shock resistance, and cost-effectiveness for your lab's needs.
Don't risk your process with the wrong equipment. Contact our technical specialists today to discuss your specific application and ensure optimal results!
Visual Guide
Related Products
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- High Purity Pure Graphite Crucible for Evaporation
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
People Also Ask
- What role does a corundum crucible play in TGA? Ensure High-Temperature Precision for Rock Sample Analysis
- What is the specific purpose of a heat-resistant crucible in a magnesium vacuum sublimation? Key Purification Insights
- Why is a high-purity platinum crucible essential for XRF of lignin ash? Ensure Precise Data with Reliable Fusion
- Why seal graphite crucibles in 316L stainless steel vessels? Ensure Precision in Molten Salt Corrosion Studies
- What role does an alumina crucible play in Al2.6Cu1.8B105 single crystal growth? Ensure High Purity and Thermal Stability
- What is the technical value of using graphite crucibles with graphite paper liners? Optimize Zr3(Al1-xSi)C2 Synthesis
- How long does a crucible last? Maximize Lifespan with Proper Material & Handling
- What is the temperature range of alumina crucibles? Key Factors for Safe High-Temp Use



















