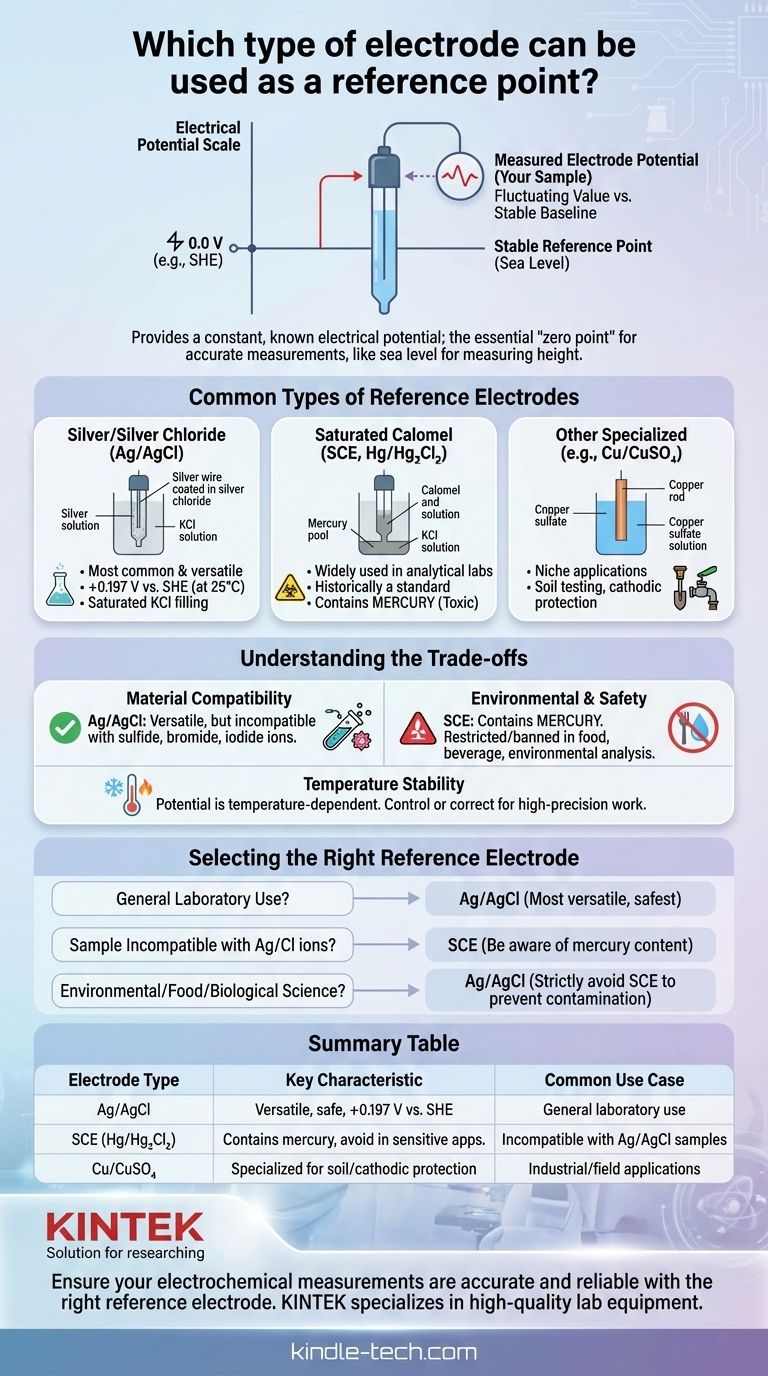
Several specialized electrodes can serve as a stable reference point in electrochemical measurements. The most common types are the Silver/Silver Chloride (Ag/AgCl) electrode and the Saturated Calomel Electrode (SCE, Hg/Hg₂Cl₂). Other electrodes, such as the Copper/Copper Sulfate (Cu/CuSO₄), are also used for specific industrial applications.
The purpose of a reference electrode is not to react with the sample but to provide a constant, known electrical potential. This stable baseline is the essential "zero point" against which the potential of another electrode—the one actually interacting with your sample—is measured.

The Role of a Stable Baseline
To understand reference electrodes, you must first understand why a stable baseline is non-negotiable for accurate measurements.
What Defines a Reference Electrode?
A reference electrode is a carefully constructed electrochemical half-cell where the components are in a stable equilibrium.
This design ensures its potential does not change significantly when small currents pass through it or as the composition of the sample solution changes.
Why a Constant Potential is Critical
Measuring an electrode's potential is like measuring a mountain's height. You need a fixed reference point, like sea level, to make any sense of the reading.
A reference electrode acts as the "sea level" for your electrical measurement. Without it, you would be measuring a fluctuating value against another fluctuating value, resulting in meaningless data.
Common Types of Reference Electrodes
While several types exist, two dominate general laboratory and field use due to their reliability and well-documented properties.
The Silver/Silver Chloride (Ag/AgCl) Electrode
This is the most common and versatile reference electrode used today.
It is based on the equilibrium between silver metal (Ag) and its salt, silver chloride (AgCl). When using a saturated potassium chloride (KCl) filling solution, it has a known potential of +0.197 V at 25°C relative to the Standard Hydrogen Electrode (SHE).
The Saturated Calomel Electrode (SCE)
The SCE is another widely used reference electrode, historically a standard in many analytical labs.
It is based on the equilibrium between mercury (Hg) and calomel (Hg₂Cl₂). It is often chosen when the sample is incompatible with the silver or chloride ions present in an Ag/AgCl electrode.
Other Specialized Electrodes
For niche applications, other systems are used. The Copper/Copper Sulfate (Cu/CuSO₄) electrode, for example, is frequently used in soil testing or for monitoring the cathodic protection of underground structures.
Understanding the Trade-offs
Choosing a reference electrode is not a one-size-fits-all decision. The right choice depends entirely on your sample and measurement goals.
Material Compatibility
The primary reason to choose one electrode over another is chemical compatibility.
An Ag/AgCl electrode is unsuitable if your sample contains ions like bromide, iodide, or sulfide, which can react with silver and alter the electrode's potential. In these cases, an SCE is a common alternative.
Environmental and Safety Concerns
This is the most significant drawback of the Calomel electrode. SCE contains mercury, a toxic heavy metal.
Because of this, its use is heavily restricted or entirely banned in applications involving food, beverages, or environmental analysis where contamination is a critical risk.
Temperature Stability
The potential of all reference electrodes is temperature-dependent. For high-precision work, it's critical to either control the temperature or record it and apply a correction factor to your results. Significant temperature fluctuations during a measurement can introduce considerable error.
Selecting the Right Reference Electrode
Your choice should be guided by your specific analytical needs, balancing performance with safety and compatibility.
- If your primary focus is general laboratory use: The Ag/AgCl electrode is the most versatile, safest, and most common starting point.
- If your sample is incompatible with silver or chloride ions: Consider a Saturated Calomel Electrode (SCE), but remain acutely aware of its mercury content and your institution's disposal policies.
- If you are working in environmental, food, or biological science: Strictly avoid mercury-based electrodes like the SCE and use the Ag/AgCl system to prevent toxic contamination.
Choosing the correct reference electrode is the foundation for obtaining reliable and repeatable electrochemical data.
Summary Table:
| Electrode Type | Key Characteristic | Common Use Case |
|---|---|---|
| Ag/AgCl | Versatile, safe, +0.197 V vs. SHE | General laboratory use |
| SCE (Hg/Hg₂Cl₂) | Contains mercury, avoid in sensitive applications | Incompatible with Ag/AgCl samples |
| Cu/CuSO₄ | Specialized for soil testing, cathodic protection | Industrial/field applications |
Ensure your electrochemical measurements are accurate and reliable with the right reference electrode. KINTEK specializes in providing high-quality lab equipment and consumables, including a full range of reference electrodes tailored to your specific application—whether you're in research, quality control, or environmental testing. Our experts can help you select the ideal electrode to prevent contamination, ensure material compatibility, and achieve precise results. Contact our team today to discuss your laboratory needs and discover how KINTEK can support your success with dependable equipment and expert guidance.
Visual Guide
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Metal Disc Electrode Electrochemical Electrode
- Copper Sulfate Reference Electrode for Laboratory Use
- Glassy Carbon Electrochemical Electrode
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
People Also Ask
- What environmental factors should be controlled when using a platinum wire/rod electrode? Ensure Accurate Electrochemical Measurements
- What are the key properties and applications of glassy carbon electrodes? | Your Guide to Superior Electrochemical Analysis
- What are the advantages of using a platinized titanium mesh? Boost Catalytic Efficiency and Durability
- What are the specific storage requirements for a sample holder? Protect Your Lab's Critical Assets
- Why is a carbon rod preferred as a counter electrode? Achieve Accurate Cyclic Polarization for FeCrNiCoNb0.5 Alloys
- Is a graphite melting point high or low? Discover Its Extreme Thermal Resilience
- How should an electrode be positioned for modification via drop-coating? Master the Upside-Down Technique
- What are the characteristics of the Iridium-Tantalum-Titanium Oxygen Evolution Electrode? High-Performance Anodes



















