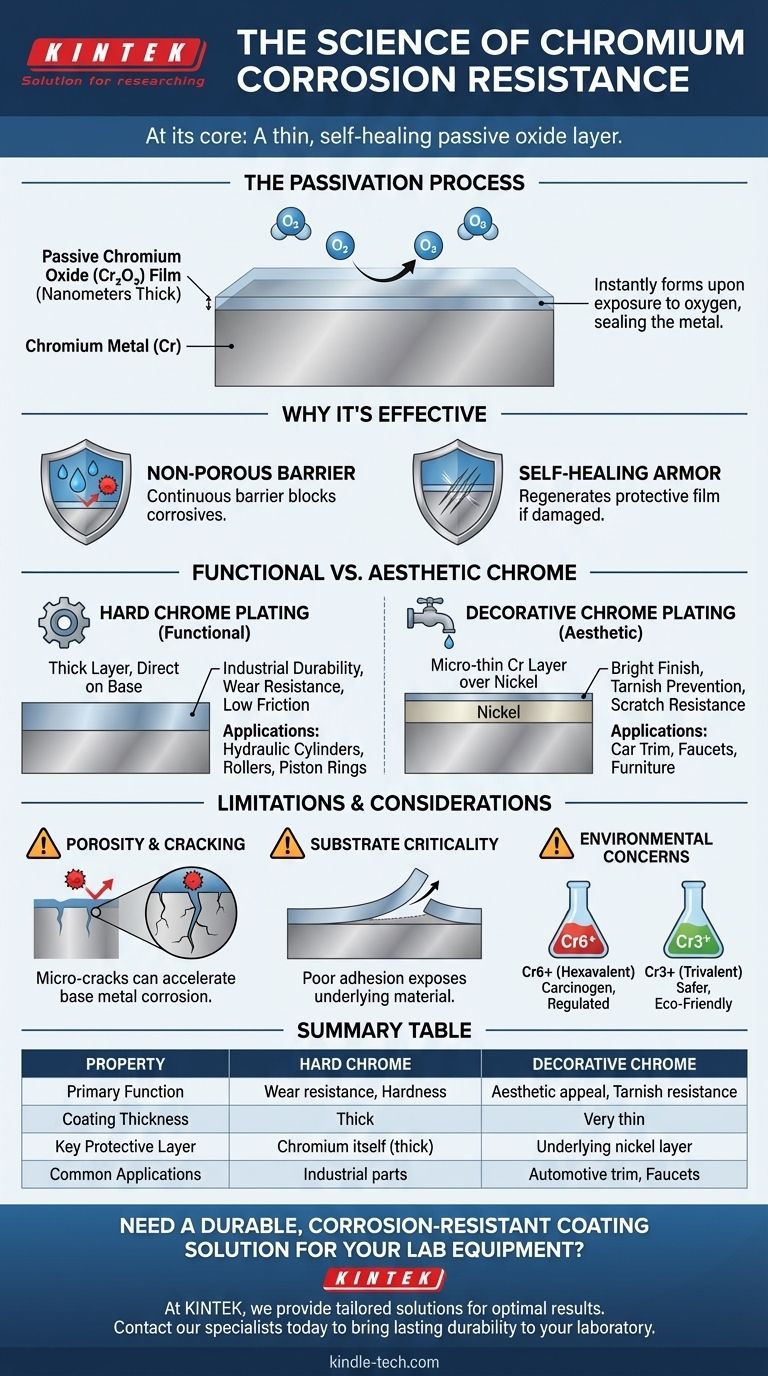
At its core, chromium's remarkable corrosion resistance comes not from being inert, but from being highly reactive. When exposed to oxygen, chromium instantly forms an extremely thin, invisible, and chemically stable layer of chromium oxide on its surface. This "passive" film acts as a durable, self-healing armor, sealing the metal underneath from environmental attack.
The key to understanding chromium is to see it not as a metal that resists corrosion, but as a metal that creates its own perfect, regenerating shield against it. This passive oxide layer is the true source of its protective power.

The Science of Passivation
The protective mechanism behind chromium is a process known as passivation. It's a microscopic phenomenon with a macroscopic impact on durability.
The Formation of the Oxide Layer
When bare chromium metal is exposed to air, it immediately reacts with oxygen. This reaction forms a molecule called chromium oxide (Cr₂O₃).
This oxide layer is incredibly thin, often only a few nanometers, making it completely transparent. It is chemically stable and non-reactive with most substances.
Why This Layer is So Effective
The chromium oxide film is not just a coating; it's a tightly bonded, integral part of the surface. It is non-porous, meaning it creates a continuous barrier that prevents water, oxygen, and other corrosive agents from reaching the chromium metal beneath.
Most importantly, the layer is self-healing. If the surface is scratched or abraded, the newly exposed chromium metal instantly reacts with oxygen again, regenerating the protective oxide film and sealing the damage.
Functional vs. Aesthetic: Not All Chrome is the Same
To leverage chromium's properties, you must understand the difference between the two primary types of chromium coating. They serve fundamentally different purposes.
Hard Chrome Plating
Hard chrome is a functional coating. It is applied in thick layers directly onto a base metal like steel to provide extreme surface hardness, low friction, and superior wear resistance.
While it offers significant corrosion protection due to its thickness, its primary purpose is industrial durability. It's used on parts like hydraulic cylinders, rollers, and piston rings.
Decorative Chrome Plating
Decorative chrome is what most people picture: the bright, mirror-like finish on car trim, faucets, and furniture. This coating is exceptionally thin.
The corrosion protection in a decorative system comes primarily from underlying layers of nickel plating. The final, micro-thin layer of chromium is added to provide a blue-white hue, prevent the nickel from tarnishing, and offer scratch resistance.
Understanding the Trade-offs and Limitations
While powerful, chromium coating is not a flawless solution. Its effectiveness is highly dependent on the quality of the application and the environment.
The Risk of Porosity and Cracking
Hard chrome plating, due to internal stresses from the plating process, naturally contains a network of micro-cracks.
If a corrosive agent penetrates these cracks and reaches the underlying base metal (e.g., steel), it can create a galvanic cell. This can accelerate corrosion of the base metal underneath the chrome plating, leading to blistering and failure.
The Critical Role of the Substrate
The protection is only as good as the bond between the chrome and the base metal. Improper cleaning, surface preparation, or plating processes can lead to poor adhesion.
If the coating peels or flakes, the underlying material is left completely exposed and the protective benefits are lost.
Environmental and Safety Concerns
The traditional and highest-performing method for chrome plating uses hexavalent chromium (Cr6+), a known carcinogen that is heavily regulated.
Trivalent chromium (Cr3+) processes are a much safer and more environmentally friendly alternative. While historically seen as less performant, modern trivalent solutions have closed the gap significantly, offering excellent corrosion resistance and aesthetics for many applications.
Making the Right Choice for Your Goal
Selecting the right chromium strategy depends entirely on your primary objective.
- If your primary focus is extreme wear resistance and industrial durability: Specify hard chrome plating, but ensure your plating partner uses exacting preparation and process controls to minimize cracking and ensure adhesion.
- If your primary focus is a bright, tarnish-proof aesthetic with good corrosion protection: A decorative chrome system, with sufficient underlying nickel layers, is the correct choice.
- If your primary focus is environmental compliance and worker safety: Prioritize sourcing from platers who use modern Trivalent chromium processes and verify that its performance meets your specific application's demands.
Ultimately, leveraging chromium effectively requires understanding that you are relying on a microscopic, self-healing shield.
Summary Table:
| Property | Hard Chrome Plating | Decorative Chrome Plating |
|---|---|---|
| Primary Function | Wear resistance, hardness | Aesthetic appeal, tarnish resistance |
| Coating Thickness | Thick | Very thin |
| Key Protective Layer | Chromium itself (thick) | Underlying nickel layer |
| Common Applications | Hydraulic cylinders, industrial parts | Automotive trim, faucets, furniture |
Need a durable, corrosion-resistant coating solution for your laboratory equipment or components?
At KINTEK, we understand the critical role that surface properties play in the longevity and performance of lab equipment. Whether you require hard chrome plating for extreme wear resistance or a decorative finish that stands up to harsh lab environments, our expertise in material science and plating technologies ensures optimal results.
We provide tailored solutions that consider your specific application demands, including compliance with environmental regulations. Let our specialists help you select the right coating to protect your investment and enhance performance.
Contact KINTEK today to discuss your lab equipment coating needs and discover how we can bring lasting durability to your laboratory.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- Custom CVD Diamond Coating for Lab Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Vertical Laboratory Tube Furnace
- Hexagonal Boron Nitride HBN Thermocouple Protection Tube
People Also Ask
- How is a high-precision electrolytic cell used to evaluate metal corrosion resistance? Validate DCT Results Accurately
- What are the advantages of a flat electrochemical cell for corrosion? Achieve Precise Pitting & Crevice Analysis
- What type of electrode system is the coating evaluation electrolytic cell designed for? Unlock Precise Coating Analysis
- What is the operating principle of a flat plate corrosion electrolytic cell? A Guide to Controlled Materials Testing
- What role does a water-jacketed electrolytic cell play in variable-temperature electrochemical corrosion measurements?














