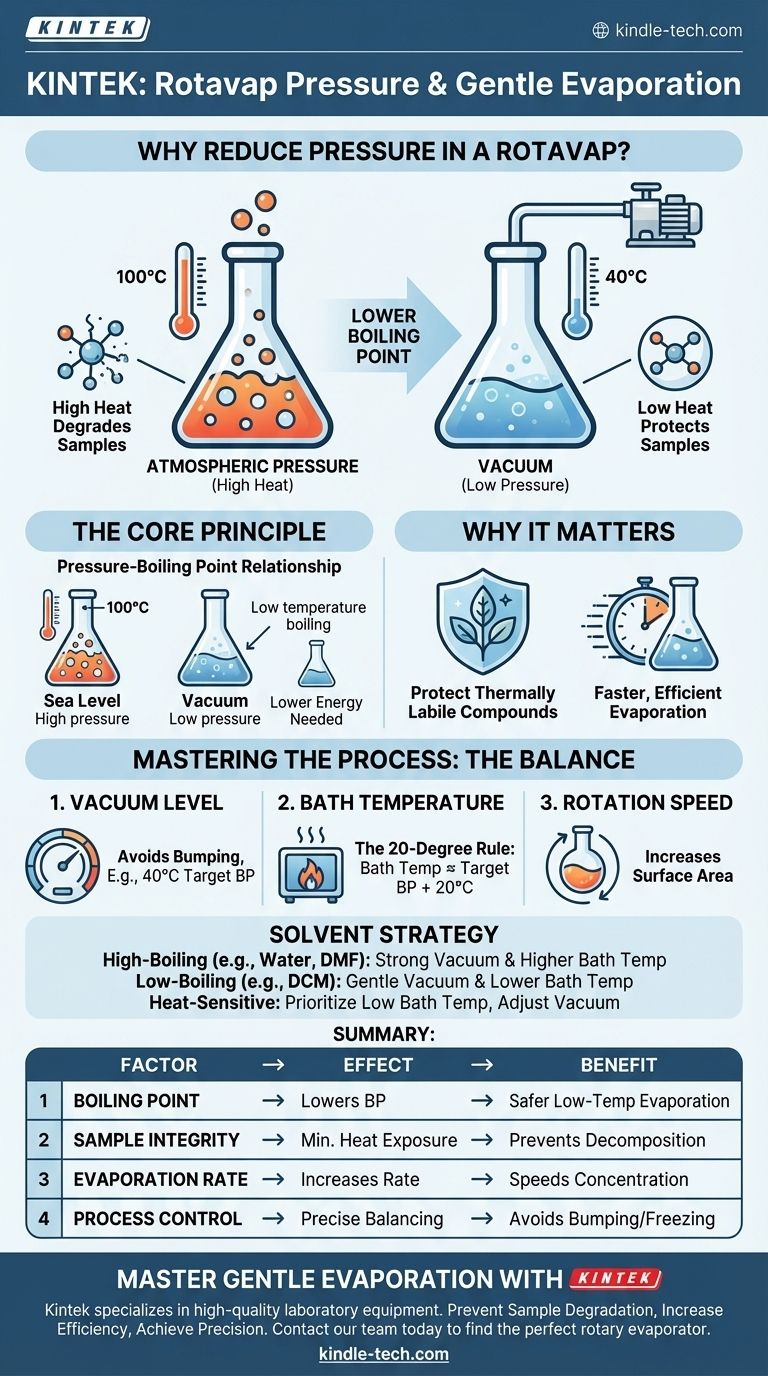
In short, reducing the pressure inside a rotary evaporator (rotovap) lowers the boiling point of your solvent. This allows you to evaporate it quickly at a much lower temperature than would be required at normal atmospheric pressure. The primary goal is to gently remove the solvent while preserving the integrity of the heat-sensitive compound dissolved within it.
A rotovap doesn't just remove a solvent; it protects your valuable sample. By applying a vacuum, you are creating an environment where the solvent can be boiled off at a low, safe temperature, preventing the high heat that would otherwise degrade or destroy your target compound.

The Fundamental Principle: The Pressure-Boiling Point Relationship
To understand the rotovap, you must first understand a core principle of physics: the boiling point of a liquid is entirely dependent on the pressure of the environment around it.
What is Boiling?
Boiling is the process where a liquid turns into a vapor. This happens when the liquid's vapor pressure—the pressure exerted by its own evaporated molecules—becomes equal to the pressure of the surrounding environment.
At sea level, the atmospheric pressure is high. For water, this means you must heat it to 100°C (212°F) to give its molecules enough energy to match that atmospheric pressure and boil.
How Vacuum Changes the Game
A vacuum pump removes air molecules from inside the rotovap, drastically lowering the environmental pressure.
With less external pressure pushing down on the surface of the solvent, the molecules need far less energy (i.e., less heat) to escape and turn into a gas. This is why water boils at a lower temperature on a high mountain, where atmospheric pressure is naturally lower. A rotovap creates an artificial "mountaintop" inside your flask.
Why This Matters: Protecting Your Sample
The entire purpose of this process is to isolate a desired compound (the solute) by removing the liquid it's dissolved in (the solvent).
The Problem with High Heat
Many compounds, especially in organic chemistry and natural product isolation, are thermally labile, meaning they are easily destroyed or altered by heat.
If you tried to boil off a solvent like ethanol (boiling point 78°C) at atmospheric pressure, that temperature could be high enough to cause your target compound to decompose, rendering your experiment a failure.
The Rotovap's Gentle Solution
By applying a vacuum, you can make that same ethanol boil at room temperature or even lower. This allows for rapid, efficient removal of the solvent without exposing your sample to damaging heat.
The rotation of the flask also plays a key role. It constantly spreads the liquid into a thin film on the inner surface, dramatically increasing the surface area for evaporation and preventing violent, uncontrolled boiling known as "bumping."
Understanding the Trade-offs and Best Practices
Simply applying the maximum possible vacuum is not always the best approach. Effective use of a rotovap involves balancing three key parameters: vacuum level, water bath temperature, and rotation speed.
Setting the Right Vacuum Level
Applying too much vacuum too quickly, especially with low-boiling-point solvents like dichloromethane (DCM) or diethyl ether, will cause violent bumping. This can splash your sample out of the flask and into the rest of the apparatus, resulting in sample loss.
A common rule of thumb is to find the pressure that lowers the solvent's boiling point to about 40°C, a safe temperature for most compounds.
Balancing with Bath Temperature
The water bath provides the energy (heat) needed for the evaporation to occur. A good guideline is the "20-degree rule": set the water bath temperature about 20°C higher than the target boiling point of your solvent at your chosen pressure.
For example, if your vacuum brings ethanol's boiling point down to 20°C, a bath temperature of 40°C is a good starting point. This provides a gentle, efficient temperature gradient to drive the evaporation.
Avoiding Solvent Freezing
A less common but possible issue is applying such a strong vacuum that the solvent's boiling point drops below its freezing point. The energy required for the rapid evaporation can cool the liquid so much that it freezes solid, stopping the process entirely. This is most common with solvents that have a relatively high freezing point, like benzene or tert-butanol.
Making the Right Choice for Your Solvent
Your strategy will depend on the properties of the solvent you need to remove.
- If your primary focus is removing a high-boiling-point solvent (like water or DMF): You will need a stronger vacuum and a higher bath temperature to provide enough energy for efficient evaporation.
- If your primary focus is removing a low-boiling-point solvent (like DCM or hexane): Apply the vacuum slowly and gently to avoid bumping, and use a correspondingly lower bath temperature.
- If your primary focus is protecting a very heat-sensitive compound: Prioritize keeping the bath temperature low (e.g., room temperature) and find the vacuum level that allows for steady evaporation at that temperature.
Mastering the interplay between vacuum and temperature transforms the rotovap from a simple machine into a precision tool for chemical separation.
Summary Table:
| Factor | Effect of Reducing Pressure | Benefit |
|---|---|---|
| Boiling Point | Lowers the boiling point of the solvent. | Enables evaporation at lower, safer temperatures. |
| Sample Integrity | Minimizes exposure to high heat. | Prevents decomposition of heat-sensitive compounds. |
| Evaporation Rate | Increases the rate of solvent removal. | Speeds up the concentration process efficiently. |
| Process Control | Allows for precise balancing of vacuum and temperature. | Prevents issues like bumping or solvent freezing. |
Master the Art of Gentle Evaporation with KINTEK
Protecting your valuable, heat-sensitive compounds during solvent removal is critical for successful outcomes. KINTEK specializes in high-quality laboratory equipment, including rotary evaporators designed for precise vacuum and temperature control.
Our solutions help you:
- Prevent Sample Degradation: Efficiently remove solvents at low temperatures to preserve compound integrity.
- Increase Efficiency: Accelerate your evaporation process with reliable and consistent performance.
- Achieve Precision: Fine-tune vacuum levels and bath temperatures for optimal results with any solvent.
Let KINTEK's expertise in lab equipment support your research and development. Contact our team today to find the perfect rotary evaporator for your laboratory's specific needs!
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- Molybdenum Tungsten Tantalum Evaporation Boat for High Temperature Applications
- Ceramic Evaporation Boat Set Alumina Crucible for Laboratory Use
- Hemispherical Bottom Tungsten Molybdenum Evaporation Boat
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
People Also Ask
- Significance of Grinding Ball Sizes and Ratios for ODS Steel? Optimize Your Milling Process for Superior Alloying
- Why are zirconia grinding jars and balls preferred for LAGP precursor powders? Ensure Purity and Ionic Conductivity
- What is the advantage of oil-free vacuum pumps regarding service life? Achieve Decades of Reliable Operation
- Why use zirconia grinding jars for fluoride solid electrolytes? Ensure High Purity & Electrochemical Stability
- What is the role of a laboratory heating system in electrolyte ohmic resistance? Optimize Precise Thermal Analysis
- What are the advantages of high-purity quartz reaction tubes compared to metal? Ensure Data Integrity in Lab Research
- Is quartz chemically resistant? Achieve Unmatched Purity and Inertness for Demanding Applications
- Why is an optical pyrometer required for B4C sintering? Ensure High-Precision Thermal Control in Hard Ceramics



















