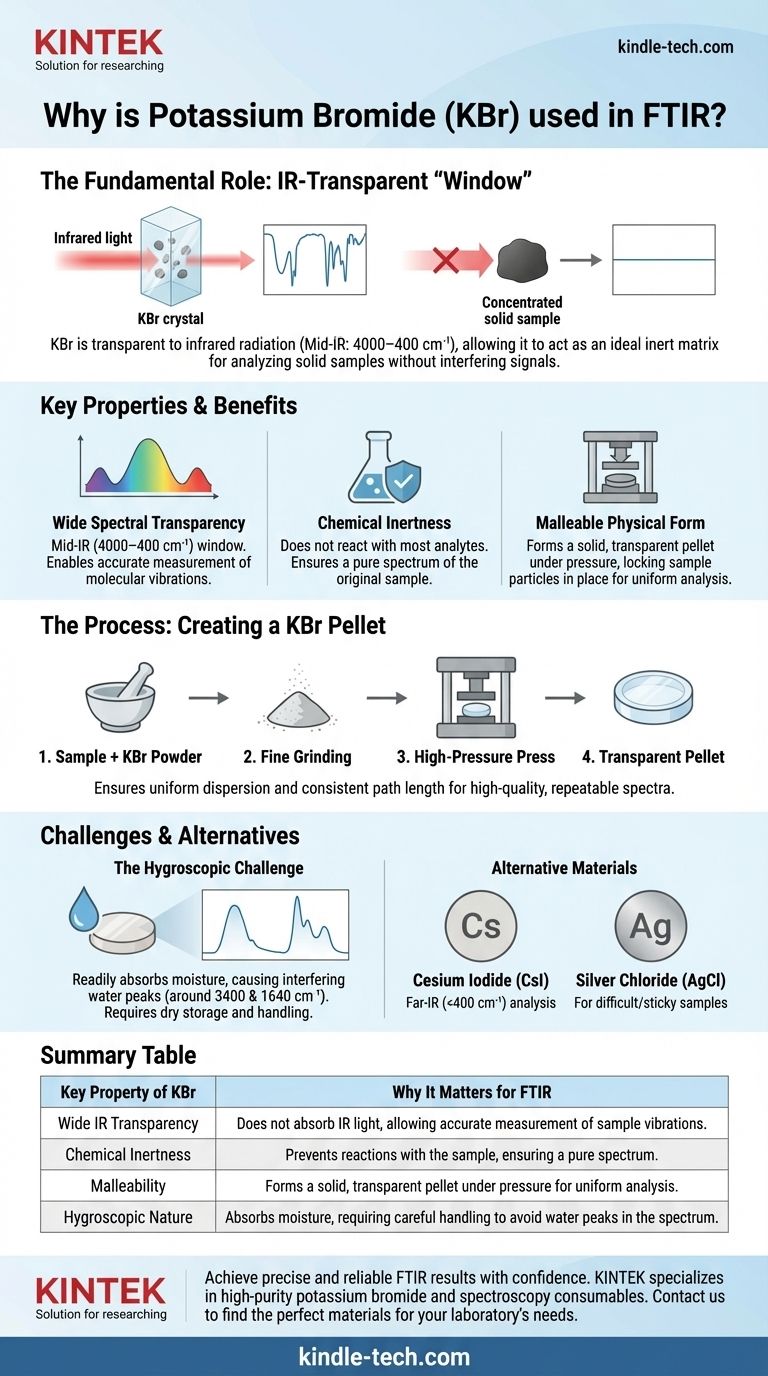
In Fourier Transform Infrared (FTIR) spectroscopy, potassium bromide (KBr) is used primarily because it is transparent to infrared radiation over the wide spectral range where molecular vibrations are measured. This property allows it to act as an ideal solid-state matrix, or "window," for holding a sample without generating its own interfering signal, ensuring the resulting spectrum belongs only to the substance being analyzed.
The core challenge with analyzing solid samples in FTIR is preparing them in a way that allows enough infrared light to pass through for a measurement. Potassium bromide serves as the perfect inert, transparent diluent, allowing you to uniformly disperse a sample in a pressed pellet that is ideal for accurate analysis.

The Fundamental Role of a Sample Matrix
The Problem of Concentrated Solids
Analyzing a solid sample in its pure, raw form is often impossible with transmission FTIR. A sample that is too thick or concentrated will absorb nearly 100% of the infrared light, resulting in a flat-lined, useless spectrum.
The goal is to get just the right amount of sample in the beam path—enough to produce clear absorption peaks but not so much that it blocks all light.
The Need for an IR-Transparent Medium
To solve the concentration problem, the sample is diluted in a medium. For this to work, the diluting medium itself must be invisible to the infrared spectrometer.
Potassium bromide is optically transparent across the entire mid-infrared region (4000–400 cm⁻¹), which is where the vast majority of characteristic molecular vibrations occur. It creates a neutral background, allowing the spectrometer to "see" only the sample.
Ensuring Uniformity and Path Length
Mixing the sample with KBr powder and pressing it under high pressure creates a thin, semi-transparent pellet. This process ensures the finely ground sample particles are distributed evenly.
This uniformity provides a consistent path length for the light beam to travel through, which is critical for producing a high-quality, repeatable spectrum with well-defined peaks.
Key Properties That Make KBr the Standard
Wide Spectral Transparency
KBr's most important feature is its lack of absorption in the mid-IR range. Its optical window extends from the ultraviolet region (~250 nm) all the way to the far-infrared (~25,000 nm, or 400 cm⁻¹), making it a versatile choice for most organic and inorganic compounds.
Chemical Inertness
An ideal matrix material must not react with the sample. KBr is an alkali halide salt that is chemically inert toward most analytes, ensuring that the spectrum you measure is of your original sample, not some unintended reaction product.
Malleable Physical Form
KBr is a relatively soft crystalline salt. When ground into a fine powder and subjected to several tons of pressure, it deforms and flows, fusing into a solid, glass-like disc that locks the dispersed sample particles in place.
Understanding the Trade-offs and Pitfalls
The Hygroscopic Challenge
The single greatest drawback of KBr is that it is hygroscopic, meaning it readily absorbs moisture from the atmosphere. Water has strong IR absorptions (a very broad peak around 3400 cm⁻¹ and another near 1640 cm⁻¹) that can easily obscure important peaks from your sample.
Because of this, KBr must be stored in a desiccator or drying oven, and sample preparation should be done in a low-humidity environment to ensure a clean background.
The Grinding Process Is Critical
For a clear pellet, both the sample and the KBr must be ground together into an extremely fine powder, typically using a mortar and pestle made of agate.
If the particle size is too large (on the order of the wavelength of the IR light), it can cause significant light scattering, which distorts the baseline of the spectrum and reduces the quality of the data.
When to Use Other Materials
While KBr is the standard, it is not the only option. For samples that need analysis in the far-infrared region (below 400 cm⁻¹), Cesium Iodide (CsI) is used, as its transparency window extends to lower frequencies. For samples that are difficult to grind or sticky, Silver Chloride (AgCl) can be used as it is much softer and can simply be pressed with the sample.
How to Apply This to Your Sample Preparation
- If your primary focus is routine analysis of a stable solid: KBr is the cost-effective, industry-standard choice, provided you properly control for moisture during storage and preparation.
- If your sample is highly sensitive to moisture or difficult to grind: Consider alternative, non-destructive techniques like Attenuated Total Reflectance (ATR-FTIR), which analyzes the surface of a sample directly.
- If you need to analyze vibrations in the far-infrared region (below 400 cm⁻¹): You must switch to a matrix material with a wider transparency window, such as Cesium Iodide (CsI).
Choosing the correct sample preparation technique is the foundation of obtaining a meaningful infrared spectrum.
Summary Table:
| Key Property of KBr | Why It Matters for FTIR |
|---|---|
| Wide IR Transparency | Does not absorb IR light, allowing accurate measurement of sample vibrations. |
| Chemical Inertness | Prevents reactions with the sample, ensuring a pure spectrum. |
| Malleability | Forms a solid, transparent pellet under pressure for uniform analysis. |
| Hygroscopic Nature | Absorbs moisture, requiring careful handling to avoid water peaks in the spectrum. |
Achieve precise and reliable FTIR results with confidence. Proper sample preparation is critical for accurate spectral data. KINTEK specializes in high-purity potassium bromide and a full range of lab equipment and consumables designed specifically for spectroscopy applications. Our products help you minimize interference and maximize clarity in your analysis. Contact us today to find the perfect materials for your laboratory's needs and let our experts support your research. Reach out via our contact form to get started!
Visual Guide
Related Products
- Customizable CO2 Reduction Flow Cell for NRR ORR and CO2RR Research
- Powerful Plastic Crusher Machine
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Laboratory High Throughput Tissue Grinding Mill Grinder
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- What is liquid phase sintering and how is it different from solid state sintering? A Guide to Faster, Denser Materials
- What is AC frame? Decoding the Two Meanings in Wi-Fi and Video
- What is the role of sintering? Transform Powder into Durable, Complex Parts
- What is the process of sputter coater? Achieve Superior Thin Film Deposition for Your Lab
- How is a constant temperature drying oven utilized in the determination of pulp yield? Ensure Precision in Biomass Data
- Why is a laboratory drying oven required for LDH powders? Achieve Precision and Structural Integrity
- What is the role of the constant flow and constant pressure pump in core flooding? Master Deep Granite Stimulation
- What are the four different types of heat treatment for metals? A Guide to Annealing, Hardening, Tempering, and Case Hardening









