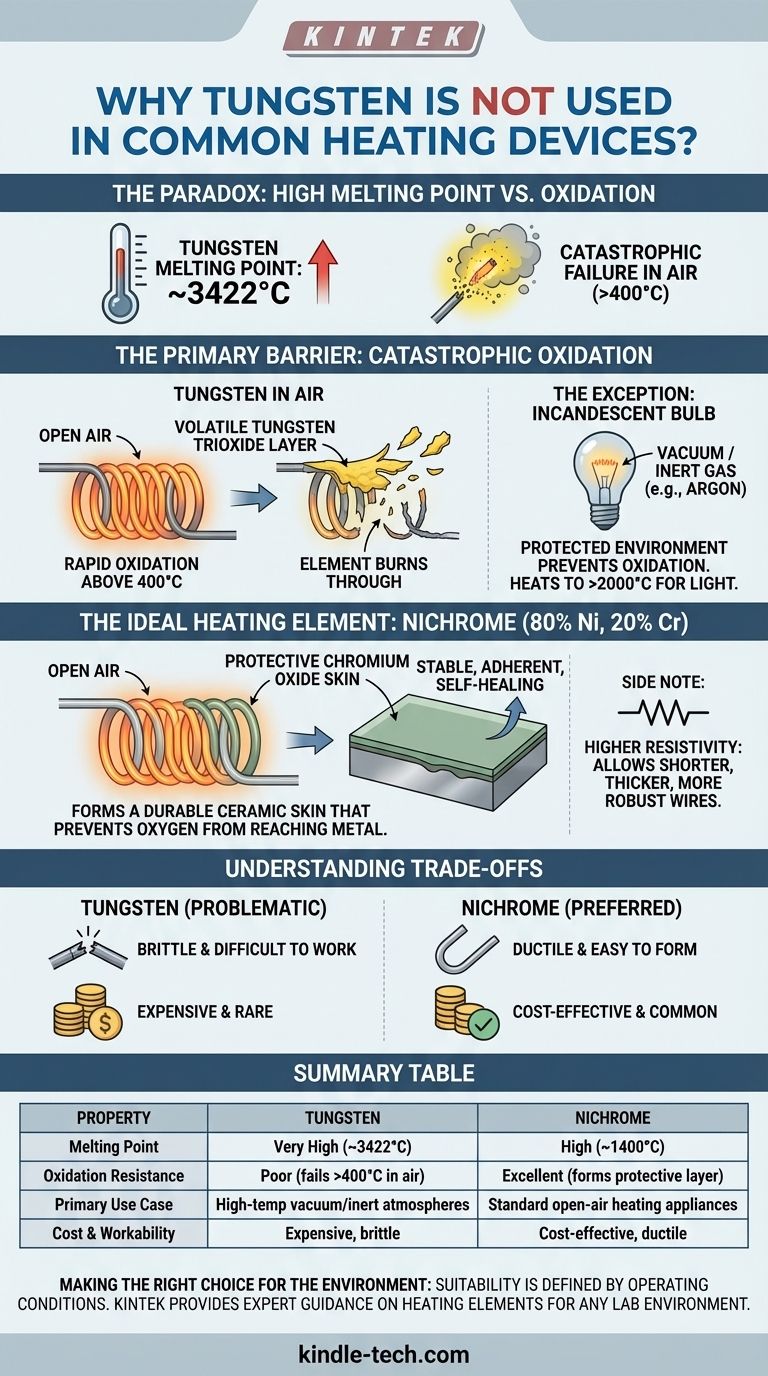
At first glance, it seems paradoxical that tungsten, the metal with the highest melting point, isn't the standard choice for the heating elements in common devices like toasters or space heaters. The primary reason is that tungsten catastrophically fails in the presence of oxygen at high temperatures. It rapidly oxidizes and evaporates, a process that would destroy a heating element in open air almost instantly.
While tungsten's ability to withstand extreme heat is unmatched, its fatal flaw is a lack of resistance to oxidation. The best materials for common heating elements are not those with the highest melting point, but those that form a stable, protective skin when heated in air.
The Primary Barrier: Catastrophic Oxidation
The single greatest factor disqualifying tungsten from use in common heating appliances is its reaction with the air around us.
How Tungsten Fails in Air
When heated above approximately 400°C (750°F), tungsten begins to react rapidly with oxygen. This process, called oxidation, forms a yellow layer of tungsten trioxide.
Unlike the stable rust that forms on iron, this oxide layer is volatile at high temperatures. It doesn't protect the underlying metal; instead, it flakes off and sublimes away, exposing fresh tungsten to be oxidized. This cycle causes the element to quickly thin out and burn through.
The Incandescent Bulb Exception
The classic incandescent light bulb is the most famous use of a tungsten filament. It works precisely because the filament is not exposed to air.
The glass bulb is either a near-perfect vacuum or, more commonly, filled with an inert (non-reactive) gas like argon. This protected environment prevents oxidation, allowing the tungsten to be heated to over 2,000°C (3,600°F) to produce brilliant light without destroying itself.
The Ideal Heating Element: The Case for Nichrome
Most heating devices use an alloy called Nichrome, which is typically composed of 80% nickel and 20% chromium. Its properties are nearly perfectly suited for producing heat in open air.
The Secret to Durability: A Protective Oxide Layer
When Nichrome is heated, the chromium in the alloy reacts with oxygen to form a thin, stable, and adherent layer of chromium oxide.
This oxide layer acts like a protective ceramic skin. It is an electrical insulator that does not flake off, and it prevents oxygen from reaching the metal underneath. If the layer is scratched, it "heals" itself by re-forming when heated again, giving the element a long and reliable service life.
The Importance of High Resistivity
A material's effectiveness as a heater depends on converting electrical energy into heat, governed by the principle P = V²/R (Power = Voltage² / Resistance).
Nichrome has a much higher electrical resistivity than tungsten. This means that for a standard household voltage, a shorter, thicker, and more robust Nichrome wire can be used to achieve the desired resistance and heat output. A tungsten wire would need to be impractically long and thin to achieve the same effect, making it fragile and difficult to manufacture.
Understanding the Trade-offs
The choice of a heating element material is a classic engineering compromise between performance, durability, and cost.
Workability and Brittleness
Tungsten is notoriously brittle at room temperature. This makes it difficult and expensive to draw into wires and form into the complex coils required for heating elements. It must be specially processed to be workable.
Nichrome, by contrast, is highly ductile. It can be easily drawn into various wire gauges and wound into coils without fracturing, which significantly simplifies the manufacturing process.
Cost and Manufacturing
Tungsten is a relatively rare element that is expensive to mine and refine. The combination of high material cost and complex processing makes it an uneconomical choice for a common appliance like a toaster or hair dryer.
Nichrome's constituent metals, nickel and chromium, are more common and the alloy is simpler to produce, making it a far more cost-effective solution for mass-market products.
Making the Right Choice for the Environment
The suitability of a material is defined entirely by its operating environment. There is no single "best" material for all heating applications; there is only the right material for the job.
- If your primary focus is generating extreme heat (>1500°C) in a vacuum or inert gas: Tungsten is the unparalleled choice due to its superior melting point and strength at high temperatures.
- If your primary focus is creating reliable, long-lasting heat in open air: An alloy like Nichrome is the definitive industry standard due to its self-protecting oxide layer and high resistivity.
Ultimately, selecting the right material is a balancing act between its intrinsic properties and the demands of its specific application.
Summary Table:
| Property | Tungsten | Nichrome (80% Ni, 20% Cr) |
|---|---|---|
| Melting Point | Very High (~3422°C) | High (~1400°C) |
| Oxidation Resistance | Poor (fails above 400°C in air) | Excellent (forms protective Cr₂O₃ layer) |
| Primary Use Case | High-temp vacuum/inert atmospheres | Standard open-air heating appliances |
| Cost & Workability | Expensive, brittle | Cost-effective, ductile |
Need a reliable heating solution for your specific application? The right material choice is critical for performance and longevity. At KINTEK, we specialize in lab equipment and consumables, providing expert guidance on heating elements for any environment—from standard open-air ovens to high-temperature vacuum furnaces. Let our specialists help you select the perfect heating technology for your laboratory's unique needs. Contact us today for a consultation!
Visual Guide
Related Products
- Thermally Evaporated Tungsten Wire for High Temperature Applications
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- High-Purity Titanium Foil and Sheet for Industrial Applications
People Also Ask
- How does a heating element heat up? The Science of Joule Heating Explained
- How does electric resistance heat work? Harnessing Direct Energy Conversion for Precise Heating
- What are the common materials used as heating elements? Find the Right Material for Your Temperature Needs
- Which is better quartz or ceramic heaters? The ultimate guide to spot vs. space heating.
- Is tungsten brittle at high temperature? Unlocking Its Extreme Heat Performance
- What is the electrical resistivity of molybdenum disilicide? Unlocking its High-Temperature Heating Power
- What causes heating element failure? Prevent Downtime by Understanding the Degradation Process
- What function do molybdenum disilicide heating elements perform? Precision Heat for Pulverized Coal Research

















