Design Criteria for Electrolytic Cells
Electrolytic Cell Volume
The volume of an electrolytic cell plays a pivotal role in determining the efficiency and accuracy of electrochemical processes. The optimal cell volume is contingent upon maintaining an appropriate ratio between the working electrode and the solution volume. This ratio is not static; it varies significantly based on the specific type of measurement or test being conducted.
For instance, in high-precision analytical measurements, a smaller cell volume with a higher electrode-to-solution ratio is often preferred. This configuration minimizes the diffusion layer thickness, thereby enhancing the sensitivity and accuracy of the readings. Conversely, in industrial applications where large-scale metal production is involved, a larger cell volume with a lower electrode-to-solution ratio might be necessary to accommodate the increased throughput and operational demands.
| Measurement Type | Preferred Cell Volume | Electrode-to-Solution Ratio |
|---|---|---|
| High-Precision Analysis | Small | High (e.g., 1:10) |
| Industrial Metal Production | Large | Low (e.g., 1:100) |
Understanding and optimizing this ratio is crucial for achieving the desired outcomes in various electrochemical applications.
Electrolytic Cell Material
When selecting materials for electrolytic cells, two primary options stand out: glass and polytetrafluoroethylene (PTFE). Each material has its own set of advantages and limitations, which must be carefully considered based on the specific application and environmental conditions.
PTFE, in particular, is renowned for its exceptional stability, especially in harsh environments such as concentrated alkali and aqua regia. This stability is crucial for maintaining the integrity and efficiency of the electrolytic process, especially in industrial settings where prolonged exposure to corrosive substances is inevitable. PTFE's inert nature ensures that it does not react with the electrolyte or other cell components, thereby minimizing potential disruptions and prolonging the cell's operational lifespan.
On the other hand, glass offers its own benefits, particularly in applications where transparency is essential for monitoring the electrolytic process. However, its susceptibility to corrosion in strong alkaline environments limits its use in certain contexts. Despite this, glass remains a popular choice for laboratory-scale experiments and educational demonstrations due to its cost-effectiveness and ease of fabrication.

In summary, the choice between glass and PTFE hinges on the specific requirements of the electrolytic process, including the chemical environment, operational longevity, and cost considerations.
Diaphragm for Electrolytic Cell
The diaphragm in an electrolytic cell serves a critical function in separating the working electrode from the auxiliary electrode. One of the most commonly used materials for this purpose is porous glass. This choice is not arbitrary; porous glass offers several advantages that enhance the performance and accuracy of the electrolytic process.
Firstly, porous glass ensures uniform current distribution throughout the cell. This uniformity is essential for maintaining consistent and reliable measurements. By providing a consistent pathway for the flow of current, porous glass helps to minimize fluctuations and errors in the data collected.
Secondly, the use of porous glass as a diaphragm reduces interference from the auxiliary electrode. In electrolytic cells, the products generated at the auxiliary electrode can sometimes diffuse into the working electrode's environment, causing unwanted reactions and skewing results. The fine pores in the glass act as a barrier, preventing such cross-contamination and preserving the integrity of the working electrode's environment.
Additionally, porous glass is highly resistant to chemical attack, making it suitable for use in a variety of electrolyte solutions. This chemical stability ensures that the diaphragm remains effective over long periods, even in harsh conditions.
In summary, the selection of porous glass as a diaphragm material is driven by its ability to provide uniform current distribution, reduce interference, and maintain chemical stability, all of which are crucial for the accurate and reliable operation of electrolytic cells.
Electrolyte
Inert electrolytes play a pivotal role in electrolytic cell design, primarily to prevent the migration of active substances that could otherwise interfere with the intended chemical reactions. The concentration of these inert electrolytes must be significantly higher than that of the active substances to ensure their effectiveness. Typically, the concentration should be at least 100 times greater than the active substance. This high concentration acts as a barrier, minimizing the potential for cross-contamination and ensuring the stability and reliability of the electrolytic process.
For instance, in industrial metal production, where precise control over chemical reactions is crucial, the use of inert electrolytes ensures that the primary reactions are not compromised by the presence of other reactive species. This high concentration ratio not only stabilizes the electrolytic environment but also enhances the efficiency of the process, reducing the likelihood of unwanted side reactions. Thus, the careful selection and management of inert electrolytes are essential components in the design and operation of electrolytic cells.
Electrolytic Cell Venting Device
Inlet and outlet channels are essential components of an electrolytic cell, designed to facilitate deoxygenation and gas adsorption processes. The configuration of these channels is critical for maintaining the efficiency and safety of the electrolytic process. Typically, the inlet is strategically placed at the bottom of the cell, ensuring that any incoming gas is evenly distributed across the electrolyte. This bottom placement aids in the thorough mixing of gases with the electrolyte, promoting effective deoxygenation.
The outlet, on the other hand, is equipped with a water seal mechanism. This water seal serves multiple purposes: it prevents the backflow of gas from the outlet, ensures a controlled release of gases, and maintains a stable pressure within the cell. By having a water seal, the electrolytic cell can operate under optimal conditions, reducing the risk of gas leakage and ensuring that the environment inside the cell remains stable and conducive to the electrolytic process.
In summary, the careful design of inlet and outlet channels, with the inlet at the bottom and the outlet featuring a water seal, is crucial for the effective deoxygenation and gas adsorption in electrolytic cells, thereby enhancing the overall efficiency and safety of the process.
Salt Bridge for Electrolytic Cell
The salt bridge is an essential component in electrolytic cells, serving as a conduit that connects the reference and research electrodes. This connection is crucial for maintaining the electrical neutrality within the cell, thereby facilitating the smooth flow of ions and completing the electrical circuit. The salt bridge is typically composed of a strong electrolyte, such as sodium chloride or potassium nitrate, which is housed in a U-shaped glass tube or soaked into porous materials like filter paper.
One of the primary functions of the salt bridge is to mitigate liquid contact potential, a phenomenon that occurs when two different electrolytes come into contact, leading to a potential difference. By ensuring that the electrolyte solution in the salt bridge is inert and nonreactive with other solutions, the risk of unwanted chemical reactions is minimized. This inertness allows for the unimpeded movement of ions between the two half-cells, maintaining a steady-state charge distribution and preventing contamination.
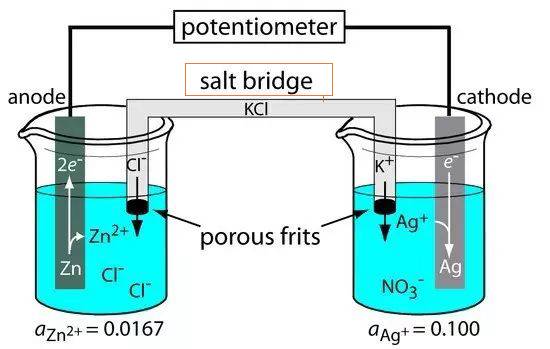
There are two common types of salt bridges: the glass tube bridge and the filter paper bridge. The glass tube bridge, as the name suggests, is a U-shaped tube filled with electrolytes, while the filter paper bridge uses porous filter paper soaked in electrolyte solutions. Both types serve the same purpose but differ in their physical structure and ease of use.
In practical applications, such as the construction of Galvanic or voltaic cells, the salt bridge plays a pivotal role. When electrons flow from one half-cell to another through an external circuit, a charge difference is established. Without the salt bridge, this charge difference would quickly halt the flow of electrons. The salt bridge allows for the continuous movement of ions, ensuring that the charge distribution remains stable and the cell operates efficiently.
In summary, the salt bridge is a critical element in electrolytic cells, enabling the seamless flow of ions and maintaining electrical neutrality. Its design and composition are carefully chosen to prevent unwanted reactions and ensure the integrity of the electrochemical process.
Rukin Capillary Tube for Electrolytic Cell
The Rukin capillary tube plays a pivotal role in electrolytic cell design, particularly in maintaining minimal resistance between the reference and working electrodes. This critical feature is essential for achieving precise potential control, which is fundamental to the accuracy and reliability of electrolytic processes.
Key Functions of the Rukin Capillary Tube
-
Minimal Resistance: The primary function of the Rukin capillary tube is to ensure that the electrical resistance between the reference and working electrodes is kept to an absolute minimum. This is achieved by the tube's design, which allows for efficient electrical conductivity without significant loss or interference.
-
Precise Potential Control: By facilitating minimal resistance, the Rukin capillary tube enables more accurate control over the potential difference between the electrodes. This precision is crucial for various electrolytic applications, from industrial metal production to scientific research, where even minor deviations in potential can lead to significant errors.
-
Enhanced Stability: The design of the Rukin capillary tube also contributes to the overall stability of the electrolytic cell. By reducing the likelihood of potential fluctuations, it helps maintain a consistent and stable environment for the electrolytic process.
Material and Design Considerations
-
Material Selection: The Rukin capillary tube is typically made from materials that offer high electrical conductivity and chemical resistance, such as platinum or gold. These materials ensure that the tube can withstand the harsh conditions of electrolytic processes without degradation.
-
Tube Geometry: The geometry of the Rukin capillary tube is carefully designed to optimize the flow of electrical current. This includes considerations of tube length, diameter, and the placement of inlet and outlet channels, all of which contribute to minimizing resistance and enhancing conductivity.
In summary, the Rukin capillary tube is an indispensable component in electrolytic cell design, offering a robust solution for ensuring minimal resistance and precise potential control. Its careful material selection and optimized design make it a key element in achieving reliable and accurate electrolytic outcomes.
Types of Electrolytic Cells
Single-Chamber Electrolytic Cell
Single-chamber electrolytic cells are specifically designed for corrosion studies, where both the research and auxiliary electrodes are housed within the same chamber. This configuration simplifies the setup by eliminating the need for complex compartmentalization, making it an ideal choice for researchers focusing on corrosion mechanisms.

In such cells, the close proximity of the research and auxiliary electrodes allows for real-time monitoring of corrosion processes. This is particularly advantageous in studies where rapid changes in electrode potential are observed, as it minimizes the time lag between measurements. Additionally, the single-chamber design reduces the risk of contamination that could arise from separate chambers, ensuring more accurate and consistent data collection.
Moreover, the use of a single chamber facilitates easier control of the electrolytic environment. Researchers can precisely adjust the electrolyte composition and concentration, as well as the temperature and pressure within the chamber, to simulate various corrosion conditions. This adaptability is crucial for understanding how different factors influence corrosion rates and patterns.
Despite its advantages, the single-chamber setup does have limitations. For instance, the lack of a diaphragm means that there is no physical barrier to separate the products of the research electrode from those of the auxiliary electrode. This can lead to potential interference in the measurements, particularly in studies involving highly reactive or volatile substances. Therefore, while the single-chamber design is practical for many corrosion studies, it may not be suitable for all experimental conditions.
Double-Chamber Electrolytic Cell
The double-chamber electrolytic cell is designed to mitigate interference from auxiliary electrode products by employing a diaphragm that separates the working electrode from the auxiliary electrode. This configuration is particularly advantageous in reducing cross-contamination and ensuring more precise measurements.

Key Features and Benefits
- Diaphragm Material: Typically constructed using porous glass, the diaphragm allows for the passage of ions while preventing the direct contact of electrode products, thereby maintaining the integrity of the working environment.
- Uniform Current Distribution: The diaphragm ensures uniform current distribution across the electrolytic cell, which is crucial for consistent and reliable results.
- Reduced Interference: By isolating the working electrode from the auxiliary electrode, the double-chamber design significantly reduces the potential for interference, enhancing the accuracy of the data collected.
Applications and Advantages
- Corrosion Studies: The double-chamber setup is particularly beneficial in corrosion studies where maintaining the purity of the working electrode is essential.
- Precision Measurements: For applications requiring high precision, such as in analytical chemistry, the double-chamber cell provides a controlled environment that minimizes external influences.
This design not only improves the accuracy of measurements but also extends the operational life of the electrodes by reducing exposure to potentially harmful byproducts.
Electrolysis Types Based on Product Generation
Electrolysis of Water Type
The electrolysis of water is a fundamental process that occurs under specific conditions, primarily involving oxygenated acids, strong bases, and oxygenated acid salt solutions of active metals. This type of electrolysis is characterized by the decomposition of water molecules into hydrogen and oxygen gases, a reaction that is both scientifically intriguing and industrially significant.
To understand the electrolysis of water, it is essential to delve into the nature of the electrolytes involved. Oxygenated acids, such as nitric acid (HNO₃) and sulfuric acid (H₂SO₄), contain oxygen atoms bonded to the central atom, which facilitates the release of oxygen during the electrolysis process. Similarly, strong bases like sodium hydroxide (NaOH) and potassium hydroxide (KOH) provide a highly alkaline environment that promotes the dissociation of water molecules.

In the context of oxygenated acid salt solutions of active metals, such as sodium nitrate (NaNO₃) or potassium sulfate (K₂SO₄), the presence of active metals like sodium (Na) or potassium (K) enhances the conductivity of the solution, thereby facilitating the electrolysis process. These salts, when dissolved in water, create a conductive medium where the active metals act as charge carriers, supporting the flow of electrons necessary for the electrolysis to occur.
The electrolysis of water is not only a key process in various industrial applications, such as the production of hydrogen gas for fuel cells, but also serves as a foundational concept in electrochemistry. Understanding the specific conditions under which this process occurs—whether in the presence of oxygenated acids, strong bases, or oxygenated acid salt solutions of active metals—is crucial for optimizing electrolytic cell design and ensuring efficient energy conversion.
Decomposition of Electrolyte Type
The decomposition of electrolyte type is a specific process that occurs under certain conditions, particularly with oxygen-free acids and solutions containing inactive metals and oxygen-free salts. This type of electrolysis is distinct from others due to the absence of oxygen, which significantly influences the chemical reactions at the electrodes.
In oxygen-free environments, the acids and salts do not contribute to the formation of oxygen gas, which is a common byproduct in many electrolysis processes. Instead, the focus is on the direct interaction between the electrolyte and the electrodes, leading to the decomposition of the electrolyte itself. This process is crucial in industrial applications where maintaining an oxygen-free environment is essential to prevent contamination and ensure the purity of the final products.
For instance, in the production of certain metals, using oxygen-free acids and salts can prevent the formation of oxides, which could otherwise compromise the quality of the metal. The absence of oxygen also simplifies the post-processing steps, as there is no need to remove oxygen-based impurities from the product.
In summary, the decomposition of electrolyte type is characterized by its reliance on oxygen-free conditions, which not only alter the chemical pathways but also enhance the efficiency and purity of the resulting products.
Hydrogen Release Alkali Type
The hydrogen release alkali type of electrolysis is a specific process that occurs in the presence of active metals and anaerobic acid solutions. This type of electrolysis is characterized by the release of hydrogen gas at the cathode, which is a direct result of the reduction of hydrogen ions present in the anaerobic acid solution. The active metals, which are typically alkali or alkaline earth metals, play a crucial role in this process by providing the necessary electrons for the reduction reaction.
The anaerobic nature of the acid solution is essential to prevent the formation of oxygen gas, which would otherwise complicate the electrolysis process. In an anaerobic environment, the hydrogen ions are the primary species that undergo reduction, leading to the efficient production of hydrogen gas. This process is particularly significant in industrial applications where the production of hydrogen gas is a key objective, such as in the production of certain chemicals or in fuel cell technology.
To facilitate this type of electrolysis, the electrolytic cell must be designed with specific considerations. The use of a diaphragm, such as porous glass, is often employed to separate the working electrode from the auxiliary electrode, ensuring uniform current distribution and minimizing interference from auxiliary electrode products. Additionally, the electrolyte concentration should be carefully controlled to prevent the migration of active substances, which could adversely affect the electrolysis process.
In summary, the hydrogen release alkali type of electrolysis is a specialized process that leverages the reactivity of active metals and the properties of anaerobic acid solutions to produce hydrogen gas efficiently. Proper electrolytic cell design and material selection are critical to ensuring the success of this process in industrial applications.
Oxygen-Generating Acid Type
The Oxygen-Generating Acid Type of electrolysis is characterized by its occurrence with inactive metal oxygenates solutions. These solutions, which include compounds such as nitrates and sulfates, are particularly significant in industrial applications where the generation of oxygen is a critical process.
In this type of electrolysis, the inactive metals, which do not readily participate in chemical reactions, play a crucial role. The oxygenates in the solution act as the primary source of oxygen, facilitating the electrolytic process. The stability of these inactive metals ensures that the electrolytic cell can operate efficiently without the risk of contamination or unintended reactions.
The process involves the decomposition of the oxygenates, leading to the release of oxygen gas at the anode. This release is a direct result of the electrolytic action, where the applied electrical current causes the oxygenates to break down into their constituent elements. The inactive metals remain largely unaffected, maintaining the integrity and purity of the electrolytic environment.
Key factors to consider in this type of electrolysis include the concentration of the oxygenates in the solution and the stability of the inactive metals. The concentration should be carefully controlled to ensure optimal oxygen generation, while the metals must be chosen based on their inertness to prevent any interference with the electrolytic process.
Overall, the Oxygen-Generating Acid Type of electrolysis is essential in industries where precise control over oxygen production is necessary, such as in metal refining and certain chemical manufacturing processes.
Energy Conversion and Electrolysis Conditions
Cathode and Anode Characteristics
In electrolytic processes, the roles of the cathode and anode can vary significantly depending on the specific reaction occurring within the cell. While these electrodes are fundamental components of the electrolytic cell, their involvement in the chemical reaction is not always straightforward.
The cathode, typically the site of reduction, and the anode, where oxidation occurs, do not necessarily participate directly in the reaction. This means that the electrodes themselves can remain chemically inert throughout the process, serving merely as conduits for the flow of electrons. For instance, in some electrolytic cells, the electrodes are made of materials like platinum or graphite, which are known for their chemical stability and resistance to reaction under the conditions of electrolysis.
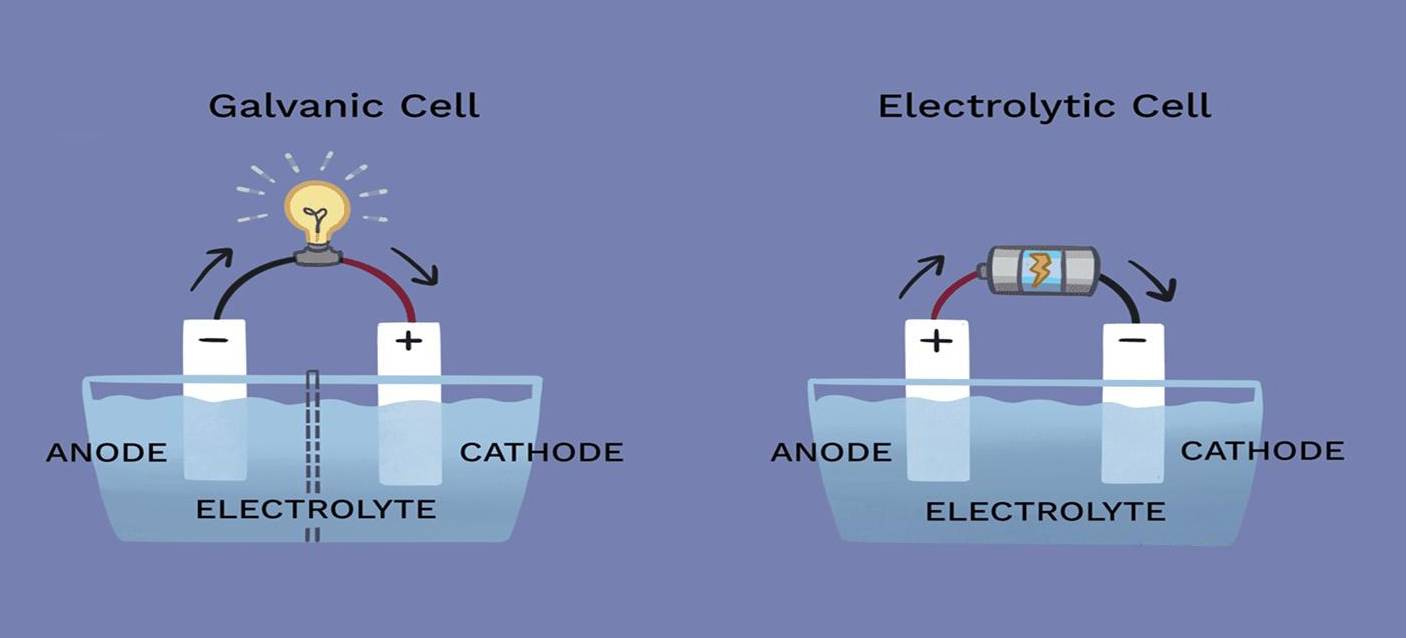
Moreover, the requirement for inertness is not universal. In certain applications, active electrodes that can undergo changes during the process are employed. These active electrodes can influence the reaction dynamics, potentially altering the efficiency or outcome of the electrolysis. Therefore, the choice between inert and active electrodes is a critical design consideration that impacts the overall performance and effectiveness of the electrolytic cell.
Conditions for Electrolysis
Electrolysis is a process that demands specific conditions to function effectively. At its core, electrolysis requires a Direct Current (DC) power supply. This ensures a consistent flow of electrical energy, which is essential for driving the chemical reactions at the electrodes.
The setup involves distinct connections for the cathode and anode. The cathode, which attracts cations, is typically connected to the negative terminal of the DC power supply, while the anode, attracting anions, is connected to the positive terminal. These connections are crucial for the proper direction of electron flow and the subsequent chemical transformations.
For the electrolysis to occur, the electrodes must be immersed in an electrolyte solution or a molten electrolyte. The electrolyte serves as the medium through which ions can move, facilitating the conduction of electricity. The choice of electrolyte is critical, as it must be capable of dissociating into ions that can participate in the electrochemical reactions.
Lastly, a closed circuit is necessary to complete the electrical pathway. This ensures that electrons can flow from the anode to the cathode through the external circuit, while ions move through the electrolyte solution. Without a closed circuit, the flow of electricity would be interrupted, and the electrolysis process would cease.
Related Products
- Flat Corrosion Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- H Type Electrolytic Cell Triple Electrochemical Cell
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
Related Articles
- Understanding Saturated Calomel Reference Electrodes: Composition, Uses, and Considerations
- Applications of Electrolytic Cells in Purification and Electroplating
- How to Make Your Own Ag/AgCl Reference Electrode for Electrochemical Experiments
- Advanced Techniques in Coating Evaluation Using Electrolytic Cells
- Understanding Electrodeposition with Electrochemical Electrodes














