Applications of XRD
Use of XRD in Material Analysis
X-ray diffraction (XRD) is a versatile technique extensively employed in the analysis of inorganic materials to elucidate critical properties such as grain size, orientation, and crystal structure. Its efficacy in phase analysis of crystalline substances is unparalleled, making it a cornerstone in material science research.
XRD operates based on Bragg’s Law, which correlates the diffraction pattern to the atomic spacing within a crystal lattice. This law enables the identification and characterization of compounds by their unique diffraction signatures. Materials can be conceptualized as a blend of ordered and disordered regions; the ordered parts, termed crystallites, exhibit regular atomic arrangements, while the disordered regions are classified as amorphous. Through XRD, the structural properties of a material can be assessed by quantifying the degree of order or disorder in the atomic configuration within the sample.
In addition to its traditional bulk analysis capabilities, XRD has evolved to include grazing incidence X-ray diffraction (GIXRD) for the characterization of thin films. GIXRD employs small incident angles, rendering the technique highly surface-sensitive. This method is particularly advantageous for probing nanometer-scale distances, as it establishes an evanescent wave that decays exponentially below the critical angle of the surface material, thereby limiting Bragg reflections to the surface structure.
| XRD Application | Description |
|---|---|
| Bulk Material Analysis | Determines grain size, orientation, and crystal structure. |
| Phase Analysis | Identifies crystalline phases through diffraction patterns. |
| Thin Film Characterization | Uses GIXRD for surface-sensitive analysis of nanometer-scale distances. |
XRD's adaptability and precision make it indispensable for comprehensive material analysis, bridging the gap between macroscopic properties and microscopic atomic arrangements.
Applications in Various Materials
X-ray diffraction (XRD) is a versatile tool in material science, offering insights into the structural and phase properties of a wide array of materials. In the realm of metallic materials, XRD is indispensable for analyzing phase transformations, alloying effects, and the integrity of crystal structures. For instance, during the synthesis of oxide phases, XRD can precisely identify the formation of different oxides and their crystalline forms, which is crucial for optimizing synthesis conditions and ensuring desired material properties.

In metal alloying, XRD plays a pivotal role in monitoring the phase changes that occur during alloy processing, such as the formation of intermetallic compounds or the segregation of elements. This information is vital for controlling the mechanical and thermal properties of the alloy, ensuring it meets specific engineering requirements.
Beyond metals, XRD is equally effective in the study of non-metallic materials. In ceramics, it aids in determining the crystalline phases present, which directly influence the material's hardness, thermal stability, and electrical conductivity. For polymers, XRD can reveal the degree of crystallinity and the arrangement of polymer chains, providing insights into the material's mechanical behavior and degradation mechanisms.
The application of XRD extends to nanomaterials as well, where it is used to characterize the size, shape, and arrangement of nanocrystals. This is particularly important in the development of advanced materials with tailored properties, such as high-strength ceramics or functional polymers. By providing detailed phase analysis and structural inspection, XRD ensures that these materials meet the stringent demands of modern technology.
Sample Preparation for XRD
Requirements for Bulk Samples
When preparing bulk samples for X-ray diffraction (XRD) testing, meticulous attention must be paid to three critical factors: surface area, cleanliness, and flatness. These attributes are essential to ensure accurate and reproducible results.
Surface Area
The surface area of the sample directly impacts the amount of material exposed to the X-ray beam, which in turn affects the quality and intensity of the diffraction pattern. A larger surface area generally provides more comprehensive data, but it must be balanced with the need for uniformity and flatness.
Cleanliness
Contaminants on the sample surface can significantly distort the diffraction pattern, leading to incorrect data interpretation. Techniques such as ultrasonic cleaning are employed to remove any surface impurities, ensuring that the sample is pristine before testing.
Flatness
The flatness of the sample is crucial for consistent XRD results. Irregularities in the sample surface can cause scattering and diffraction anomalies. To achieve the necessary flatness, techniques like grinding and polishing are used to prepare metal blocks, thin films, and sheet specimens. These methods help to create a uniform, planar surface that is optimal for XRD analysis.
In summary, the preparation of bulk samples for XRD requires a combination of grinding, polishing, and ultrasonic cleaning to meet the stringent requirements of surface area, cleanliness, and flatness. These steps are vital to ensuring that the XRD data is accurate and reliable.
Requirements for Powder Samples
Preparing powder samples for X-ray diffraction (XRD) testing demands meticulous attention to detail, particularly in grinding and sieving processes. The primary objective is to achieve a uniform particle size distribution, typically requiring the powder to be ground to a 320 mesh size. This fine grinding ensures that the particles are small enough to produce clear diffraction patterns, which are crucial for accurate phase analysis and structural inspection.
However, the process is not without its challenges. Over-grinding can lead to the formation of amorphous particles, which significantly alters the diffraction patterns and compromises the accuracy of the analysis. Therefore, it is essential to strike a balance between achieving a fine particle size and avoiding excessive grinding that could induce amorphization.

To mitigate these risks, pre-treatment steps such as grinding and sieving are employed. These steps help in homogenizing the particle size distribution and ensuring that the sample is well-prepared for XRD testing. The sieving process further refines the particle size, removing any larger particles that could skew the diffraction results.
In summary, the preparation of powder samples for XRD involves a delicate balance of grinding and sieving to achieve the desired particle size without inducing amorphization. This meticulous approach ensures that the sample is optimally prepared for accurate and reliable XRD analysis.
Filming Methods
When preparing powder samples for X-ray diffraction (XRD) analysis, two primary filming methods are commonly employed: the smear method and the press method. Each method has its distinct advantages and is suited to different sample sizes and requirements.
The smear method is particularly advantageous for handling small sample sizes. This technique involves spreading a thin, even layer of powder directly onto the sample holder. The smear method is ideal for samples that are difficult to obtain in large quantities, ensuring that even minimal amounts of material can be effectively analyzed. This method also allows for quick and easy application, making it a practical choice for initial assessments or when time is a constraint.
On the other hand, the press method is designed to ensure a flat and uniform plane of sample powder. This technique involves pressing the powder into a flat surface using specialized tools, such as a die and press. The press method is particularly useful for larger sample sizes and provides a more consistent and reproducible surface for XRD analysis. By ensuring a flat plane, this method minimizes irregularities that could affect the accuracy of diffraction data, making it a preferred choice for detailed and precise analyses.
In summary, while the smear method is suited for small sample sizes and quick applications, the press method ensures a flat and uniform surface, ideal for larger samples and more precise XRD analysis.
Data Analysis in XRD
Diffraction Angle and Crystal Planes
Determining the crystal planes corresponding to diffraction angles in X-ray diffraction (XRD) is a critical step in material analysis. This process typically involves matching the observed diffraction patterns with standard powder diffraction data cards, which provide a comprehensive database of known crystal structures and their corresponding diffraction angles. For materials with known structures, this matching process can be straightforward, allowing researchers to quickly identify the crystal planes responsible for the observed diffraction peaks.
For unknown or complex structures, however, the task becomes more challenging. In such cases, specialized software tools like treaor90 can be invaluable. These tools employ advanced algorithms to analyze diffraction patterns, taking into account various factors such as peak intensity, width, and position. By comparing the experimental data with a vast library of known crystal structures, these software solutions can assist in identifying the most likely crystal planes, even when the material's structure is not immediately apparent.
Moreover, the use of such software is not limited to identifying crystal planes. They can also provide insights into other aspects of the diffraction data, such as the influence of sample grain size on peak width and intensity. This holistic approach ensures that the analysis is comprehensive, covering all relevant aspects of the diffraction pattern.
In summary, while standard powder diffraction data cards are essential for identifying crystal planes in known structures, specialized software like treaor90 plays a crucial role in unraveling the mysteries of unknown or complex materials. This combination of traditional methods and modern computational tools ensures that XRD remains a powerful and versatile technique in material science research.
Diffraction Intensity and Peak Width
Diffraction intensity and peak width are critical parameters in X-ray diffraction (XRD) analysis, primarily influenced by the sample's grain size. The size and distribution of these grains significantly impact the scattering patterns observed in XRD spectra. Fine grinding of the sample is essential to achieve optimal diffraction results. This process enhances scattering, as smaller grains provide more surface area for X-rays to interact with, leading to sharper and more intense peaks in the diffraction pattern.
However, there is a delicate balance to maintain. Over-grinding the sample can have detrimental effects. Excessive grinding can lead to amorphization, a state where the crystalline structure of the material is disrupted, resulting in a loss of long-range order. This amorphization manifests as broadened peaks in the XRD pattern, complicating the interpretation of the data. The broadening of these peaks obscures the distinct diffraction features, making it difficult to accurately determine the crystal structure and grain size.
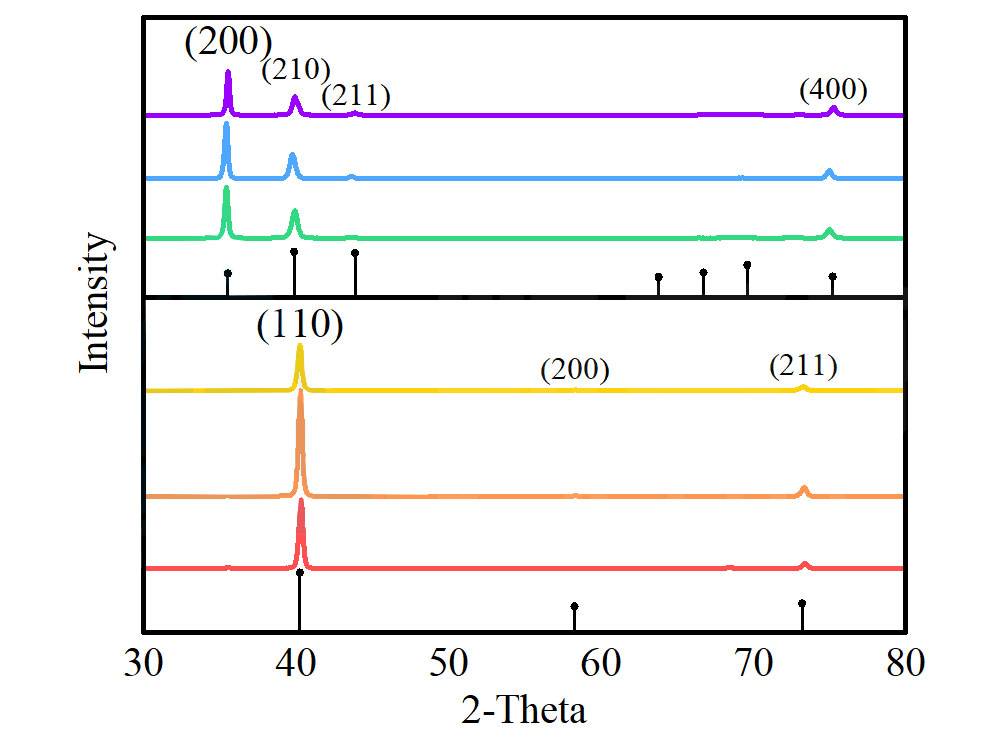
To avoid these issues, it is crucial to monitor the grinding process closely. The goal is to achieve a fine, uniform grain size without inducing amorphization. Techniques such as sieving and controlled grinding can help maintain the desired grain size distribution. Additionally, using standard samples for calibration can aid in identifying any broadening effects due to over-grinding, ensuring more accurate and reliable XRD data analysis.
Peak Shifts in XRD
Peak shifts in X-ray diffraction (XRD) patterns can be indicative of several underlying issues, each contributing to variations in the diffraction angles. One primary cause is element substitution, where the replacement of one element with another within the crystalline structure alters the lattice parameters, leading to shifts in peak positions. This phenomenon is particularly common in alloys and composite materials, where different elements may occupy similar lattice sites.
Another significant factor is sample preparation errors. Improper grinding or sieving of powder samples can result in non-uniform particle sizes, leading to inconsistent diffraction patterns. For instance, over-grinding can cause amorphization, where the crystalline structure is disrupted, and this can manifest as peak shifts. Similarly, bulk samples that are not properly polished or cleaned can introduce surface irregularities that affect diffraction angles.
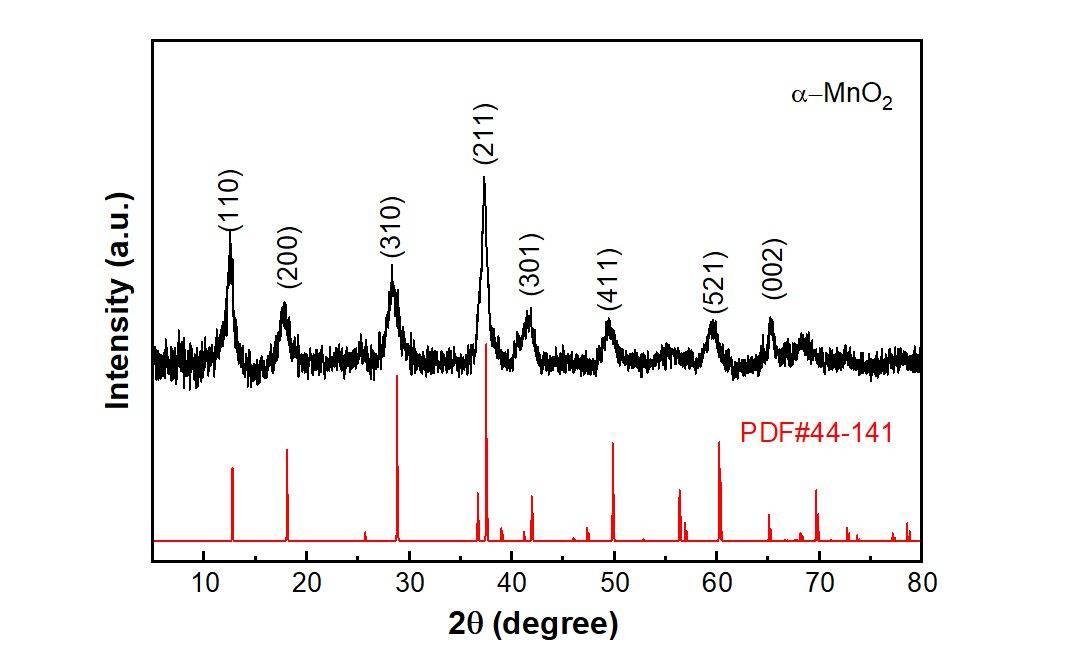
Instrument calibration issues also play a crucial role in peak shifts. Calibration errors can arise from misalignment of the XRD instrument or the use of outdated calibration standards. To mitigate these issues, it is essential to regularly calibrate the instrument using standard samples that have known diffraction patterns. These standards serve as a reference to correct any deviations in the measured data, ensuring accurate and reliable results.
In summary, understanding the causes of peak shifts is vital for accurate data interpretation in XRD. By addressing element substitution, refining sample preparation techniques, and maintaining rigorous instrument calibration, researchers can minimize these shifts and enhance the precision of their XRD analyses.
Related Products
- XRD Sample Holder X-ray Diffractometer Powder Slide
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- XRF & KBR plastic ring lab Powder Pellet Pressing Mold for FTIR
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- XRF Boric Acid Lab Powder Pellet Pressing Mold for Laboratory Use
Related Articles
- XRF Pelletising for Solid Samples Tips and Tricks
- Unlocking the Power of XRF Spectrometer Modules: A Comprehensive Guide
- What is xrf analysis and how to making pressed xrf pellets
- How To Turn XRF analysis sample preparation Into Success
- Dilution Ratios for XRF Pelletising Finding the Optimal Balance