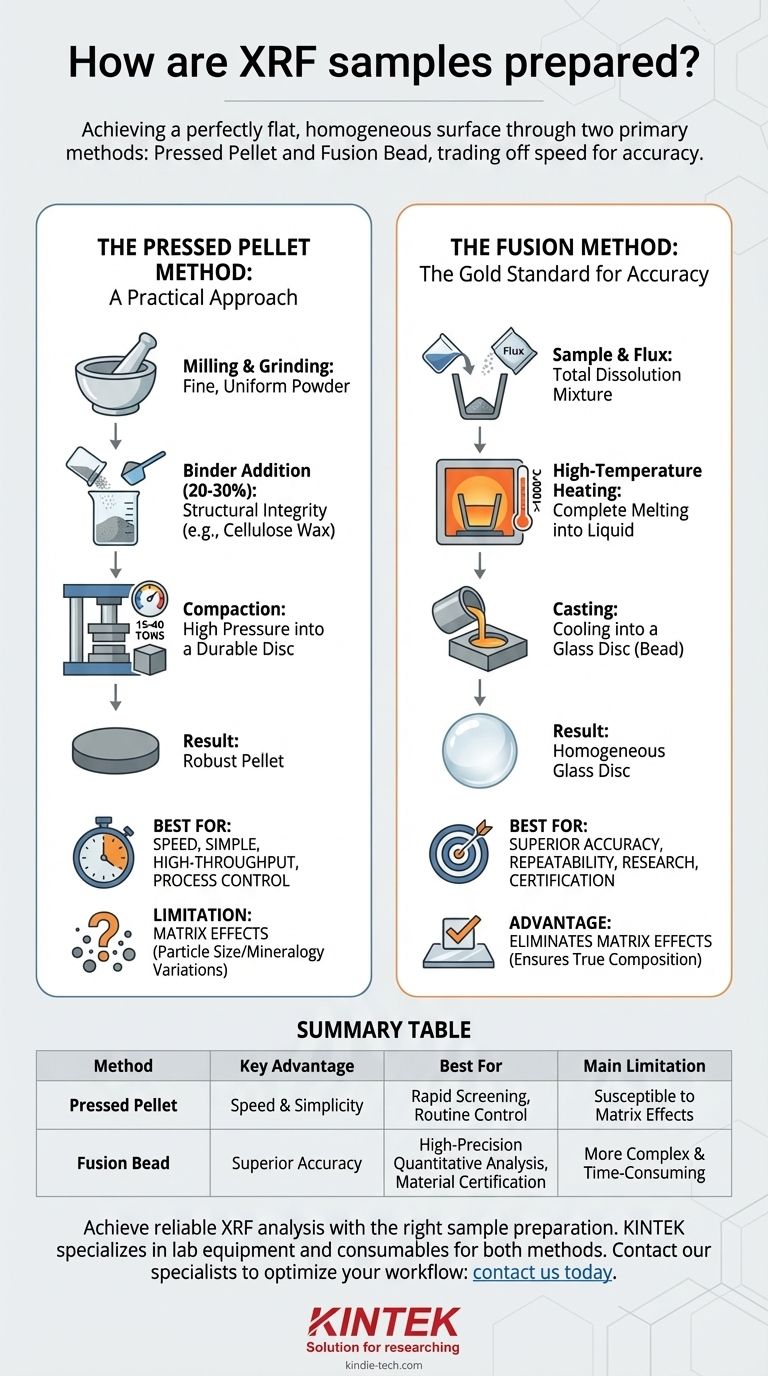
To prepare a sample for X-ray Fluorescence (XRF) analysis, you must transform it into a perfectly flat, homogeneous surface. The two primary industry-standard methods for achieving this are creating a pressed powder pellet or a fused glass bead, each with distinct advantages depending on your analytical needs.
The core decision in XRF sample preparation is a trade-off between speed and accuracy. The pressed pellet method is fast and straightforward for many applications, while the fusion method is more complex but provides superior accuracy by eliminating physical and chemical inconsistencies in the sample.

The Pressed Pellet Method: A Practical Approach
This technique involves compressing a finely ground sample powder into a solid, durable disc. It is often chosen for its speed and simplicity, making it ideal for high-throughput environments.
The Goal: Creating a Homogeneous Powder
The first and most critical step is milling or grinding the raw sample. The objective is to reduce the material to a fine, uniform powder, which minimizes analytical errors caused by inconsistent particle sizes.
The Role of the Binder
A binding agent, such as a cellulose wax powder, is then thoroughly mixed with the sample. The binder provides the structural integrity needed to form a robust pellet that won't crumble during handling or analysis.
A binder-to-sample ratio of 20-30% is a common starting point, but this can be optimized to reduce sample dilution while maintaining pellet strength.
The Compaction Process
The powder mixture is placed into a pellet die and compressed under high pressure, typically between 15 and 40 tons.
It is crucial to release this pressure slowly. Releasing it too quickly can cause microscopic cracks to form on the pellet's surface, compromising the integrity of the sample and the accuracy of the final analysis.
The Fusion Method: The Gold Standard for Accuracy
For applications demanding the highest level of precision, the fusion method is the preferred technique. It eliminates the physical inconsistencies inherent in powder samples by completely dissolving the material.
The Goal: Total Sample Dissolution
This process involves mixing the sample with a lithium borate salt, known as a flux. This mixture is then heated in a platinum or zirconium crucible to temperatures exceeding 1000°C.
At this temperature, the sample completely dissolves into the molten flux, creating a perfectly homogeneous liquid solution.
Creating the Glass Disc
The molten mixture is then agitated and poured into a mold to cool. The result is a stable, uniform glass disc (or "bead") with a flawless surface, perfectly suited for highly accurate and repeatable XRF analysis.
Understanding the Trade-offs
Choosing the correct method requires understanding the balance between analytical requirements, sample type, and operational efficiency.
Speed vs. Accuracy
Pressed pellets are significantly faster to prepare, often taking only a few minutes. This makes them suitable for process control or screening applications where speed is paramount.
Fusion beads require a more involved, high-temperature process, but they yield far more accurate and repeatable results. This is essential for research, quality certification, and the analysis of unknown materials.
The Problem of "Matrix Effects"
The primary source of error in the pressed pellet method is known as matrix effects. These are inaccuracies caused by variations in particle size, mineralogy, and surface finish within the sample.
The fusion method's greatest strength is its ability to completely eliminate matrix effects. By dissolving the sample into a glass, it removes all physical variables, ensuring the analysis reflects only the sample's true elemental composition.
Making the Right Choice for Your Analysis
Your analytical goal is the most important factor in selecting a sample preparation technique.
- If your primary focus is rapid screening or routine process control: The pressed pellet method offers the necessary speed and sufficient accuracy for these tasks.
- If your primary focus is high-accuracy quantitative analysis or material certification: The fusion method is the only way to eliminate matrix effects and achieve the highest level of data reliability.
- If you are analyzing materials with complex or varied mineralogies: Fusion is strongly recommended to create a truly homogeneous sample and avoid misleading results.
Ultimately, proper sample preparation is the foundation of any reliable XRF analysis.
Summary Table:
| Method | Key Advantage | Best For | Main Limitation |
|---|---|---|---|
| Pressed Pellet | Speed and simplicity | Rapid screening, routine process control | Susceptible to matrix effects (particle size/mineralogy variations) |
| Fusion Bead | Superior accuracy and repeatability | High-precision quantitative analysis, material certification | More complex and time-consuming process |
Achieve reliable XRF analysis with the right sample preparation. The choice between pressed pellets and fusion beads is critical for your lab's accuracy and efficiency. KINTEK specializes in lab equipment and consumables for both methods, providing the presses, dies, fluxes, and crucibles you need. Our expertise ensures you get consistent, high-quality results, whether you prioritize speed or ultimate precision. Let our specialists help you optimize your XRF workflow—contact us today for a consultation!
Visual Guide
Related Products
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- XRF Boric Acid Lab Powder Pellet Pressing Mold for Laboratory Use
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- Laboratory Manual Hydraulic Pellet Press for Lab Use
People Also Ask
- What is molding technique? A Guide to High-Volume, Complex Part Manufacturing
- What are the hazards of power press? Protect Your Team from Crushing and Amputation Risks
- How do you use a KBr press? Master the Art of Creating Transparent Pellets for FTIR Analysis
- What is a hydraulic forging press used for? Harnessing Controlled Power for Complex Metal Forming
- How powerful is hydraulic pressure? Generate Immense Force for Heavy-Duty Applications
- What is KBr pellet used in the examination of? Mastering FTIR Spectroscopy for Solid Samples
- What can a hydraulic press be used for? From Industrial Forging to Lab Analysis
- How is a laboratory hydraulic press used to evaluate the stability of solid biopesticide formulations? Optimize Pellets



















