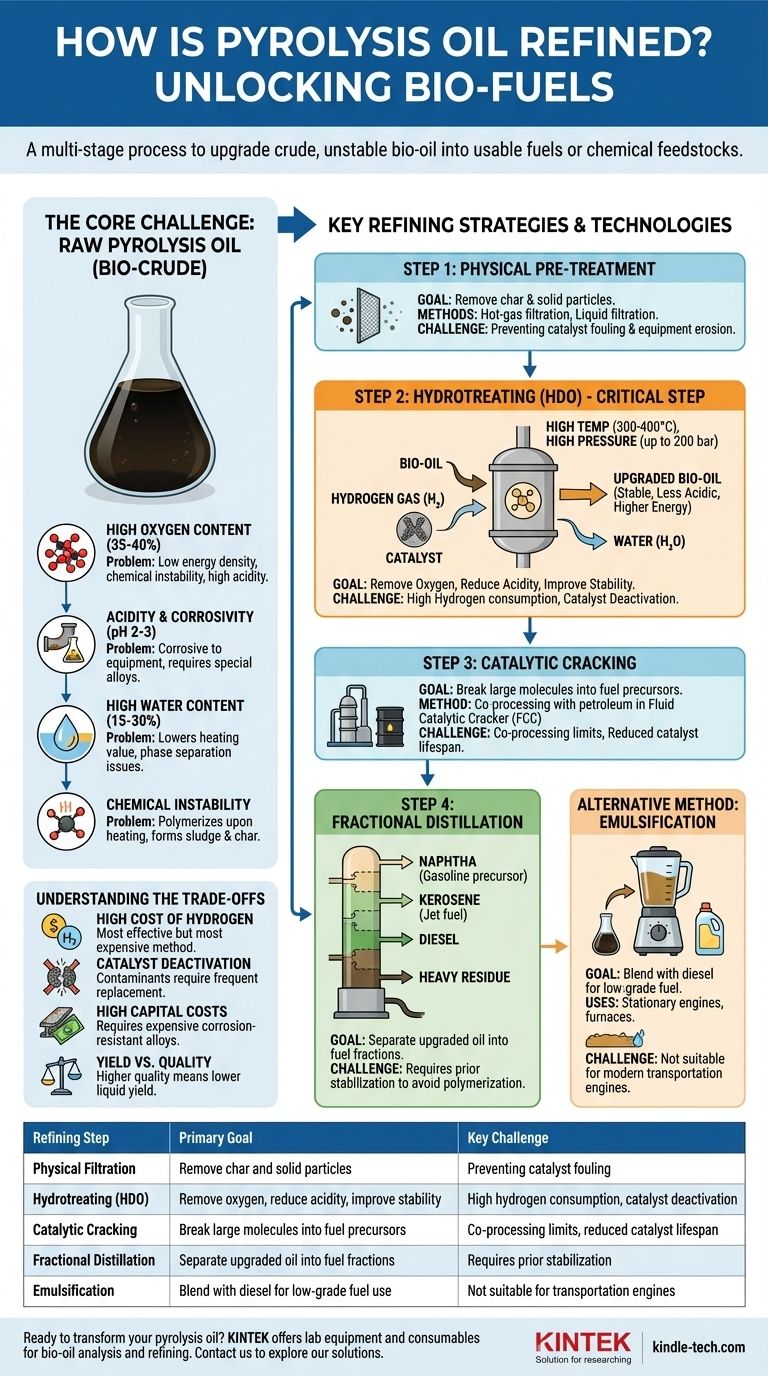
In short, refining pyrolysis oil is a multi-stage process required to upgrade the crude, unstable bio-oil into usable fuels or chemical feedstocks. The primary methods involve physical filtration to remove char, followed by chemical upgrading—most critically, hydrotreating (or hydrodeoxygenation) to remove oxygen, reduce acidity, and improve stability. Subsequent steps like fractional distillation can then be used to separate the upgraded oil into specific fuel cuts, similar to conventional crude oil refining.
The core challenge of refining pyrolysis oil is not just purification, but a fundamental chemical transformation. The goal is to remove the high concentration of oxygen, which makes the oil acidic, corrosive, and unstable, in order to create a hydrocarbon-like product compatible with existing fuel infrastructure.

The Core Challenge: Why Raw Pyrolysis Oil Is Unusable
Raw pyrolysis oil, often called bio-oil or bio-crude, is fundamentally different from conventional crude oil. Its unique chemical properties necessitate aggressive refining before it can be used as a "drop-in" fuel.
High Oxygen Content
Unlike petroleum, which is composed almost entirely of hydrocarbons (hydrogen and carbon), pyrolysis oil contains a significant amount of oxygen (35-40% by weight). This oxygen is bound within compounds like acids, aldehydes, ketones, and phenols.
This high oxygen content is the root cause of most other problems, as it leads to low energy density, chemical instability, and high acidity.
Acidity and Corrosivity
The oxygenated compounds, particularly acetic and formic acids, make pyrolysis oil highly acidic, with a pH typically between 2 and 3. This makes the oil extremely corrosive to standard carbon steel pipes, tanks, and engine components, requiring specialized and expensive corrosion-resistant materials.
High Water Content
Pyrolysis oil can contain 15-30% water, which is produced during the pyrolysis process and is miscible with the oil. This water content significantly lowers the oil's heating value and can lead to phase separation issues during storage or processing.
Chemical Instability
Pyrolysis oil is thermally unstable. When heated, its reactive oxygenated compounds tend to polymerize, forming thick sludge and solid char. This makes traditional refining processes like distillation nearly impossible without prior stabilization, as the oil will solidify and clog equipment at elevated temperatures.
Key Refining Strategies and Technologies
Refining pyrolysis oil involves a sequence of steps designed to systematically address its inherent problems. No single method is a complete solution; they are often used in combination.
Step 1: Physical Pre-Treatment
The essential first step is removing physical contaminants. This typically involves hot-gas filtration to remove char particles directly after the pyrolysis reactor and/or liquid filtration of the condensed oil. This prevents downstream catalyst fouling and equipment erosion.
Step 2: Hydrotreating (Hydrodeoxygenation - HDO)
This is the most critical and effective refining technology for pyrolysis oil. The process involves reacting the oil with hydrogen gas at high temperatures (300-400°C) and pressures (up to 200 bar) in the presence of a catalyst.
The primary goal of HDO is to remove oxygen atoms by converting them into water (H₂O). This simultaneously reduces acidity, increases the oil's heating value, and dramatically improves its chemical stability. The resulting product is a more hydrocarbon-like liquid that is far less corrosive.
Step 3: Catalytic Cracking
Once stabilized, the upgraded bio-oil can potentially be co-processed in a standard refinery's Fluid Catalytic Cracker (FCC). Here, it is blended in small amounts (typically <5%) with petroleum gas oil.
The FCC unit "cracks" the large molecules into smaller, more valuable ones like gasoline. However, co-processing bio-oil still presents challenges, including reduced catalyst lifespan and lower fuel yields compared to processing pure petroleum streams.
Step 4: Fractional Distillation
After significant hydrotreating, the upgraded oil becomes stable enough to withstand the high temperatures of distillation. Fractional distillation separates the oil into different fractions based on their boiling points, such as naphtha (a gasoline precursor), kerosene (jet fuel), and diesel.
Direct distillation of raw pyrolysis oil is not feasible due to its tendency to polymerize and coke.
Alternative Method: Emulsification
For less demanding applications, emulsification is a lower-cost upgrading option. This involves blending pyrolysis oil with a conventional fuel like diesel and adding a surfactant package. The result is a stable emulsion that can be burned in some stationary engines, furnaces, or boilers, though it is not suitable for modern transportation engines.
Understanding the Trade-offs
Upgrading pyrolysis oil is technically feasible, but it comes with significant economic and engineering challenges that must be carefully considered.
The High Cost of Hydrogen
Hydrotreating is the most effective method, but it is also the most expensive. It consumes large quantities of hydrogen, which is a costly industrial gas to produce and handle. This hydrogen consumption is the single largest operational expense in upgrading bio-oil to fuel.
Catalyst Deactivation
The residual contaminants and acidic nature of pyrolysis oil, even after pre-treatment, are harsh on the catalysts used in HDO and cracking. These catalysts lose their effectiveness (deactivate) quickly, requiring frequent and costly replacement or regeneration.
High Capital Costs
The corrosive nature of raw and partially upgraded bio-oil requires that reactors, piping, and vessels be constructed from expensive alloys like stainless steel. Furthermore, the high-pressure, high-temperature conditions of hydrotreating demand robust and expensive reactor systems.
Yield vs. Quality
There is an inherent trade-off between the quality of the final product and the liquid yield. More aggressive refining (higher temperatures, longer residence times) produces a higher-quality, fully deoxygenated oil but also converts more of the liquid into light gases, reducing the overall volume of liquid fuel produced.
Making the Right Choice for Your Goal
The optimal refining strategy depends entirely on your target end-product and economic constraints.
- If your primary focus is producing transportation-grade drop-in fuels: A multi-stage pathway involving robust filtration, deep hydrotreating, and fractional distillation is the only viable route.
- If your goal is to generate lower-grade fuel for stationary boilers or furnaces: A simpler process of filtration followed by emulsification with diesel may be a sufficient and more cost-effective solution.
- If you aim to extract high-value biochemicals: A combination of solvent extraction and vacuum distillation on specific, mildly upgraded oil fractions may be prioritized over complete deoxygenation for fuel.
Ultimately, transforming raw pyrolysis oil into a valuable product depends on a clear-eyed assessment of its challenging properties and a strategic investment in the right refining technology.
Summary Table:
| Refining Step | Primary Goal | Key Challenge |
|---|---|---|
| Physical Filtration | Remove char and solid particles | Preventing catalyst fouling and equipment erosion |
| Hydrotreating (HDO) | Remove oxygen, reduce acidity, improve stability | High hydrogen consumption and catalyst deactivation |
| Catalytic Cracking | Break large molecules into fuel precursors | Co-processing limits and reduced catalyst lifespan |
| Fractional Distillation | Separate upgraded oil into fuel fractions (e.g., diesel, naphtha) | Requires prior stabilization to avoid polymerization |
| Emulsification | Blend with diesel for low-grade fuel use | Not suitable for transportation engines |
Ready to transform your pyrolysis oil into high-value products? KINTEK specializes in lab equipment and consumables for bio-oil analysis, upgrading, and refinement. Whether you're researching hydrotreating catalysts, optimizing filtration, or scaling distillation processes, our solutions help you tackle corrosion, instability, and efficiency challenges. Contact our experts today to explore how we can support your laboratory's pyrolysis oil refining goals!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
- High Performance Laboratory Freeze Dryer
People Also Ask
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds



















