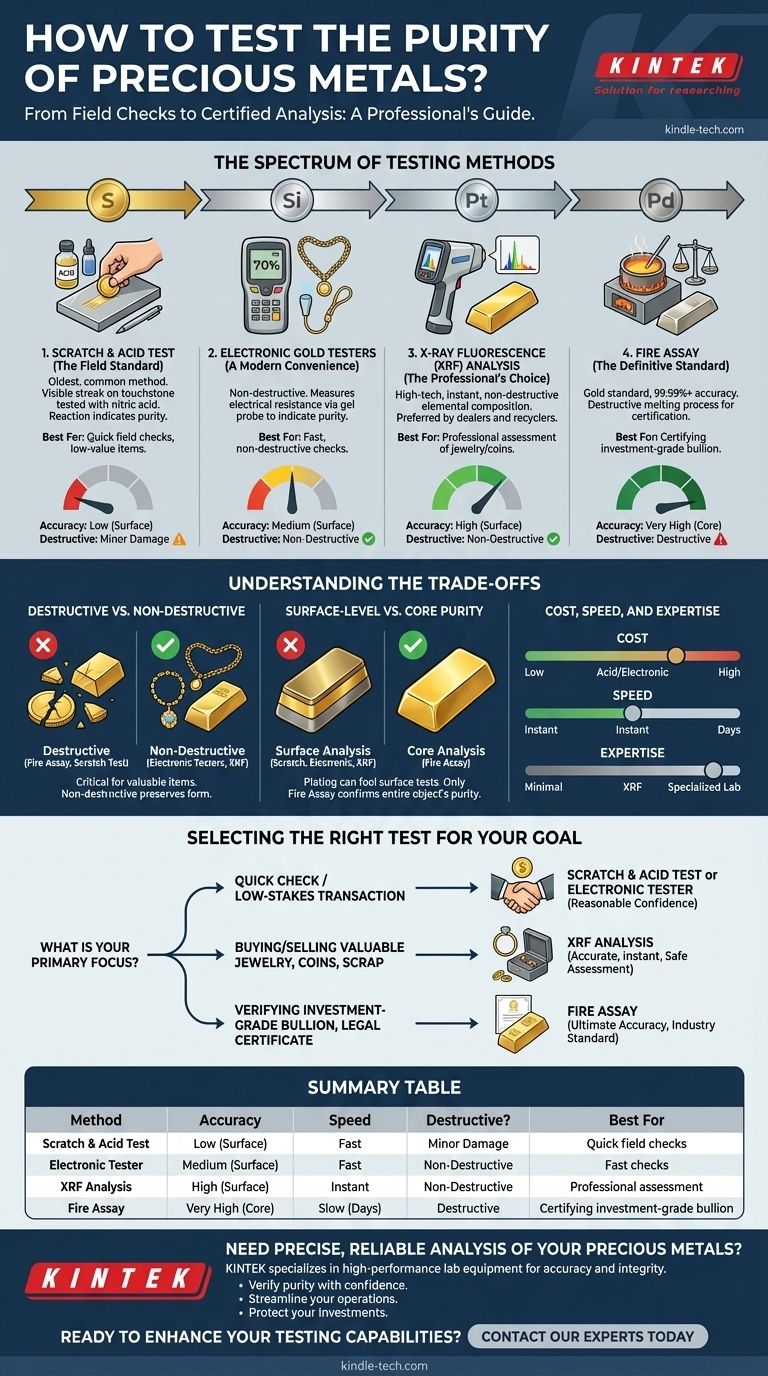
To test the purity of precious metals, professionals use a range of methods from simple chemical tests to highly advanced lab analyses. The most common techniques include the scratch and acid test for a quick estimate, electronic testers for a non-destructive check, X-Ray Fluorescence (XRF) for a precise surface analysis, and fire assay for the most accurate, definitive, but destructive measurement.
The core decision is not which test is "best," but which one provides the right balance of accuracy, cost, and potential damage for your specific goal. A quick field test is suitable for low-value items, while investment-grade material demands a definitive, certified analysis.

The Spectrum of Testing Methods
Choosing a testing method involves navigating a spectrum from quick, accessible checks to slower, more expensive, but far more accurate laboratory procedures. Each has its place depending on the value of the item and the certainty required.
The Scratch and Acid Test (The Field Standard)
This is the oldest and most common method used by jewelers and pawnshops. A small sample of the metal is rubbed onto a black touchstone, creating a visible streak.
Drops of nitric acid of varying strengths are then applied to the streak. The reaction of the acid—or lack thereof—indicates the metal's karatage or purity. For example, acid formulated for 14k gold will dissolve a 10k streak but leave a 14k streak untouched.
Electronic Gold Testers (A Modern Convenience)
Electronic testers offer a non-destructive alternative to acids. These handheld devices pass a small electrical current through the metal via a gel probe.
The device measures the electrical resistance of the metal, which corresponds to a specific purity level, and displays the result digitally. They are fast and relatively easy to use but require careful calibration and can sometimes be fooled by thick gold plating.
X-Ray Fluorescence (XRF) Analysis (The Professional's Choice)
An XRF analyzer is a high-tech instrument that provides a rapid and highly accurate elemental composition of an item without causing any damage. It is the preferred method for most reputable bullion dealers and precious metal recyclers.
The device bombards the metal with low-level X-rays, causing the atoms in the sample to emit fluorescent X-rays at energy levels unique to each element. The analyzer detects these emissions and instantly reports the exact percentages of gold, silver, platinum, and other alloying metals present.
Fire Assay (The Definitive Standard)
Fire assay is the gold standard for determining purity and is used by mints and refineries to certify investment-grade bullion. It is the most accurate method available but is also completely destructive.
The process involves taking a carefully weighed sample of the metal and melting it in a furnace with lead and other reagents. This separates the precious metals from the base metals. The resulting pure metal bead is then weighed, and its weight is compared to the original sample's weight to calculate the exact purity, often to 99.99% accuracy or higher.
Understanding the Trade-offs
No single method is perfect for every scenario. Your choice depends on a critical balance between certainty, cost, and the preservation of your item.
Destructive vs. Non-Destructive
This is the most critical consideration for valuable items. A fire assay will destroy the coin, bar, or piece of jewelry being tested. A scratch test creates permanent, albeit minor, physical damage.
In contrast, electronic testers and XRF analyzers are completely non-destructive, making them ideal for assessing finished goods like jewelry, rare coins, or any item where preserving its form is essential.
Surface-Level vs. Core Purity
A major weakness of simpler tests is that they only analyze the surface of an item. A thickly plated piece of brass can easily fool a basic scratch test and sometimes even an electronic tester.
An XRF analyzer provides a more penetrating analysis but is still primarily a surface-level reading. Only a fire assay, which consumes the entire sample, can give you absolute certainty about the purity of the entire object, not just its outer layer.
Cost, Speed, and Expertise
Acid testing kits are inexpensive but require practice to interpret correctly and involve handling hazardous chemicals. Electronic testers are more expensive upfront but are faster and safer for repeated use.
XRF analyzers represent a significant investment, often costing tens of thousands of dollars, placing them in the hands of serious professionals. Fire assay is performed by specialized labs for a fee and can take days to produce a result.
Selecting the Right Test for Your Goal
Your reason for testing determines the appropriate method.
- If your primary focus is a quick check for a low-stakes transaction: A scratch and acid test or a calibrated electronic tester provides a reasonable degree of confidence.
- If your primary focus is buying or selling valuable jewelry, coins, or scrap: Insist on a test from a professional using a non-destructive XRF analyzer to get an accurate, instant, and safe assessment.
- If your primary focus is verifying investment-grade bullion or needing a legally defensible purity certificate: A fire assay from an accredited laboratory is the only method that meets the industry standard for ultimate accuracy.
Ultimately, empowering yourself with knowledge of these methods ensures you can verify the true value of your precious metals with confidence.
Summary Table:
| Method | Accuracy | Speed | Destructive? | Best For |
|---|---|---|---|---|
| Scratch & Acid Test | Low (Surface) | Fast | Minor Damage | Quick field checks, low-value items |
| Electronic Tester | Medium (Surface) | Fast | Non-Destructive | Fast, non-destructive checks |
| XRF Analysis | High (Surface) | Instant | Non-Destructive | Professional assessment of jewelry/coins |
| Fire Assay | Very High (Core) | Slow (Days) | Destructive | Certifying investment-grade bullion |
Need precise, reliable analysis of your precious metals?
For professionals who demand accuracy and integrity, the right equipment is essential. KINTEK specializes in high-performance lab equipment, including advanced analyzers perfect for the precious metals industry.
We help you:
- Verify purity with confidence using reliable, cutting-edge technology.
- Streamline your operations with fast, accurate, and non-destructive testing solutions.
- Protect your investments by ensuring every item is correctly assessed.
Ready to enhance your testing capabilities? Contact our experts today to find the perfect solution for your laboratory's needs.
Visual Guide
Related Products
- Platinum Sheet Electrode for Battery Lab Applications
- Small Lab Rubber Calendering Machine
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Laboratory High Pressure Horizontal Autoclave Steam Sterilizer for Lab Use
- Platinum Sheet Electrode for Laboratory and Industrial Applications
People Also Ask
- What are the advantages of electron beam method? Achieve Speed, Cost Savings, and Material Integrity
- How does plasma sputtering work? Master Precision Thin-Film Deposition
- What is the best prevention for CVD? A Lifelong Strategy for Optimal Heart Health
- How does a high-speed homogenizer prepare m-BN and PNF dispersions? Achieve Uniform Molecular-Level Integration
- What are the different types of ash test? Choose the Right Method for Your Material
- What is the fastest method of quenching? Achieve Maximum Hardness with Agitated Brine
- What are the key issues in the synthesis of nanomaterials? Overcoming Size, Shape, and Purity Control Challenges
- What are 3 advantages of an electric furnace? Lower Cost, Safer Operation & Universal Availability










