The process of creating a CVD diamond is a method of atomic-level construction rather than geological force. It begins with a thin diamond "seed" placed in a vacuum chamber, which is then heated to extreme temperatures and filled with a carbon-rich gas. This gas is energized into a plasma, causing carbon atoms to rain down and bond to the seed, growing a real diamond layer by layer over several weeks.
Unlike methods that mimic the earth's immense pressure, Chemical Vapor Deposition (CVD) 'grows' a diamond in a low-pressure chamber. It works by systematically depositing carbon atoms from a superheated gas onto a diamond seed, building the crystal one atomic layer at a time.

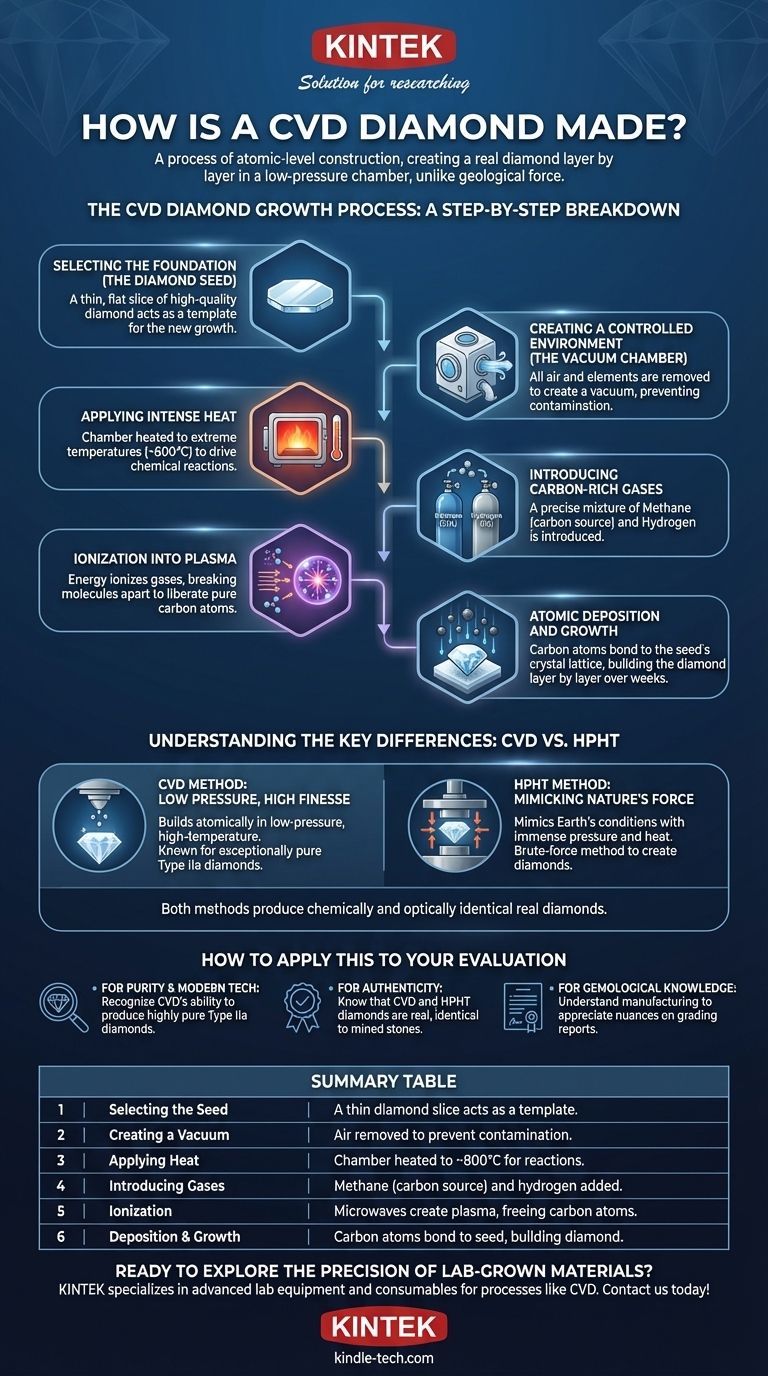
The CVD Diamond Growth Process: A Step-by-Step Breakdown
To truly understand a CVD diamond, you must understand the meticulous, highly controlled process behind its creation. Each step is critical to forming a flawless crystal structure identical to that of a mined diamond.
Step 1: Selecting the Foundation (The Diamond Seed)
The entire process starts with a diamond seed. This is a very thin, flat slice of a previously grown high-quality diamond.
This seed acts as the template, providing the foundational crystal lattice upon which the new diamond will grow.
Step 2: Creating a Controlled Environment (The Vacuum Chamber)
The diamond seed is carefully cleaned and placed inside a sealed, high-tech chamber.
All air and other elements are pumped out to create a vacuum. This step is crucial to prevent any contamination from interfering with the diamond’s pure carbon structure during growth.
Step 3: Applying Intense Heat
The chamber is heated to an extremely high temperature, typically around 800 degrees Celsius.
This intense heat provides the energy needed to drive the chemical reactions that will follow.
Step 4: Introducing Carbon-Rich Gases
A precise mixture of gases, primarily methane and hydrogen, is introduced into the chamber.
Methane (CH4) serves as the source for the carbon atoms, while hydrogen plays a critical role in purifying the process and preventing the formation of lesser forms of carbon, like graphite.
Step 5: Ionization into Plasma
Energy, often in the form of microwaves, is used to ionize the gases. This process strips the molecules of their electrons, creating a glowing ball of plasma inside the chamber.
This superheated plasma cloud effectively breaks the gas molecules apart, which liberates the pure carbon atoms from the methane.
Step 6: Atomic Deposition and Growth
The freed carbon atoms are drawn down to the slightly cooler diamond seed at the bottom of the chamber.
They bond to the seed's crystal lattice, perfectly replicating its structure. This layer-by-layer deposition slowly builds the new diamond, a process that typically takes two to four weeks to produce a sizeable gemstone.
Understanding the Key Differences: CVD vs. HPHT
CVD is one of two primary methods for creating lab-grown diamonds. The other, High Pressure/High Temperature (HPHT), uses a fundamentally different approach.
The CVD Method: Low Pressure, High Finesse
As described, the CVD process is one of deposition. It builds a diamond atomically in a low-pressure, high-temperature environment.
This method is renowned for its ability to produce exceptionally pure diamonds (known as Type IIa), which are very rare in nature.
The HPHT Method: Mimicking Nature's Force
The HPHT method mimics the conditions deep within the Earth. It takes a carbon source (like graphite) and subjects it to immense pressure and heat, essentially squeezing it into a diamond.
This brute-force method was the original way lab diamonds were created and is still widely used today. Both methods produce diamonds that are chemically and optically identical to their natural counterparts.
How to Apply This to Your Evaluation
Understanding the manufacturing process is not just an academic exercise; it empowers you to be a more informed buyer and appreciate the technology involved.
- If your primary focus is on purity and modern technology: Recognize that the CVD process is a cutting-edge method renowned for producing highly pure Type IIa diamonds.
- If your primary focus is on authenticity: Know that whether a diamond is made via CVD or HPHT, the result is a real diamond with the same physical, chemical, and optical properties as one mined from the earth.
- If your primary focus is on gemological knowledge: Differentiating between the two growth methods helps you understand the nuances on a grading report and the story behind the stone's creation.
Ultimately, understanding the CVD process reveals the remarkable technological control required to construct a diamond atom by atom.
Summary Table:
| Step | Process | Key Details |
|---|---|---|
| 1 | Selecting the Seed | A thin slice of a high-quality diamond acts as a template. |
| 2 | Creating a Vacuum | Air is removed to prevent contamination in the chamber. |
| 3 | Applying Heat | Chamber is heated to ~800°C to drive chemical reactions. |
| 4 | Introducing Gases | Methane (carbon source) and hydrogen are added. |
| 5 | Ionization | Microwaves create a plasma, freeing carbon atoms. |
| 6 | Deposition & Growth | Carbon atoms bond to the seed, building the diamond over weeks. |
Ready to explore the precision of lab-grown materials? KINTEK specializes in advanced lab equipment and consumables, including the technology behind processes like CVD. Whether you're in research, gemology, or materials science, our solutions provide the reliability and control you need. Contact us today to learn how we can support your laboratory's innovative work!
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- CVD Diamond for Thermal Management Applications
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
People Also Ask
- How can nanotubes be used as a catalyst? Enhance Performance and Durability of Metal Catalysts
- What is the purpose of ITO coating? Enabling Transparent Electronics for Modern Devices
- What do the optical properties of thin film depend on? Mastering Material, Thickness, and Process
- What are carbon nanotubes explain its types? Unlocking the Power of SWCNTs and MWCNTs
- What are the methods of graphene production? Top-Down vs. Bottom-Up for Your Lab's Needs
- What are the advantages of ion beam sputtering? Achieve Superior Thin Film Quality and Precision
- What is the evaporation process in semiconductors? A Guide to Thin Film Deposition
- What are the advantages and applications of electronic thin films? Precision Engineering for High-Efficiency Design



















