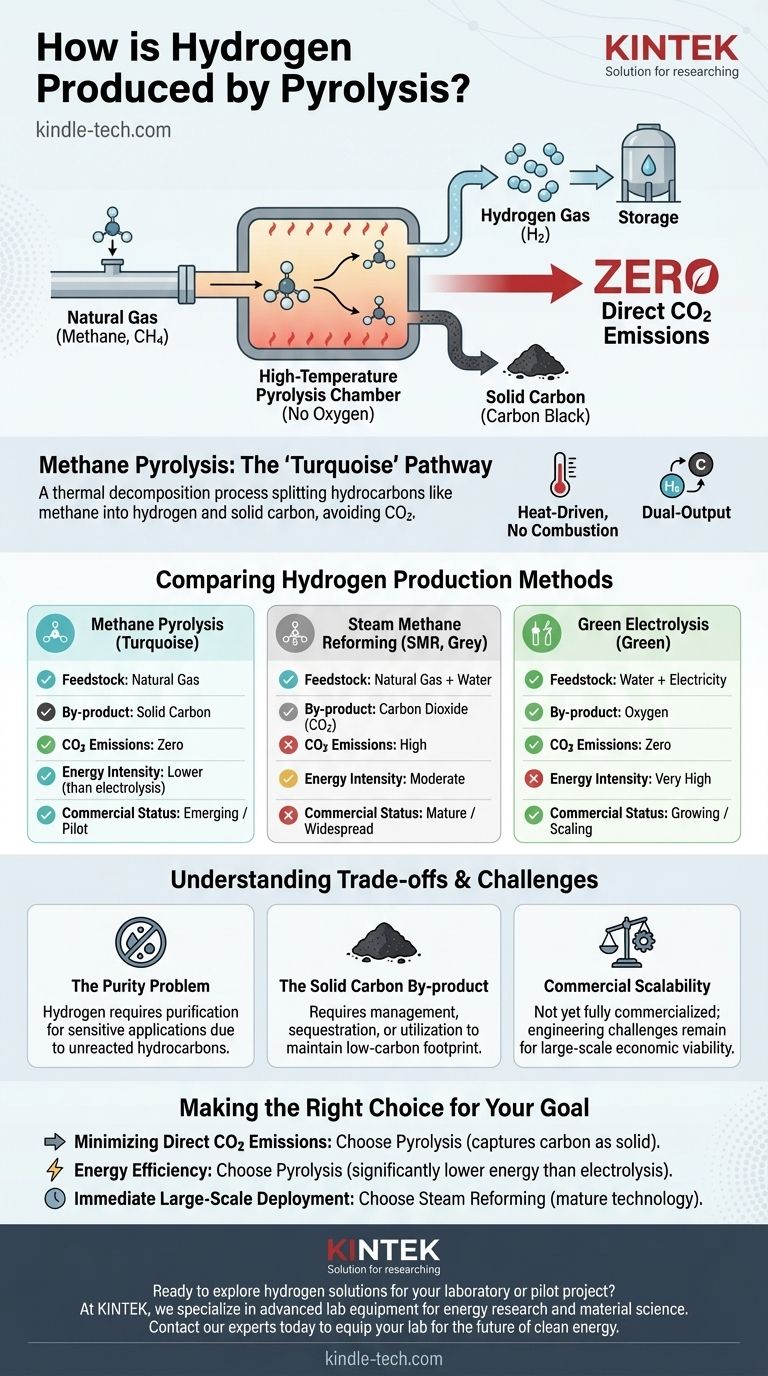
In short, hydrogen is produced by pyrolysis when a hydrocarbon source, most commonly natural gas (methane), is heated to high temperatures in an environment without oxygen. This thermal decomposition process cracks the methane molecule (CH4), splitting it into its constituent parts: hydrogen gas (H2) and solid carbon (C), effectively avoiding the creation of carbon dioxide (CO2).
Methane pyrolysis represents a "turquoise" hydrogen production pathway. It offers a compelling middle ground between traditional, high-emission steam reforming and energy-intensive green electrolysis by producing low-carbon hydrogen and a useful solid carbon by-product instead of CO2.

The Core Mechanism of Methane Pyrolysis
The Fundamental Chemical Reaction
The process is fundamentally a thermal decomposition. One molecule of methane (CH4) is broken down by heat into one atom of solid carbon (C) and two molecules of hydrogen gas (2H2).
The Critical Role of Heat
Pyrolysis is not combustion. By heating natural gas without the presence of oxygen, the molecular bonds are broken apart without burning, which prevents the carbon from combining with oxygen to form CO2.
The Two Key Outputs
This process yields two distinct and valuable products. The primary product is hydrogen gas, and the by-product is solid carbon, often called carbon black.
How Pyrolysis Compares to Other Methods
Methane Pyrolysis vs. Steam Reforming
Steam Methane Reforming (SMR) is the current industry standard. SMR reacts methane with water vapor, producing more hydrogen per molecule of methane but also creating one molecule of CO2 as a direct by-product.
Pyrolysis, in contrast, creates zero direct CO2 emissions. Its primary by-product is solid carbon, which must be managed separately.
Methane Pyrolysis vs. Green Hydrogen (Electrolysis)
Green hydrogen is produced by using electricity to split water (H2O) into hydrogen and oxygen. While completely free of carbon emissions, this process is extremely energy-intensive.
Methane pyrolysis requires significantly less energy. Some methods can produce hydrogen using as little as one-eighth of the energy required for electrolysis, making it a more energetically favorable process.
Understanding the Trade-offs and Challenges
The Purity Problem
The hydrogen gas produced through pyrolysis is not pure. It contains unreacted hydrocarbons and other impurities that must be removed through additional gas purification steps before it can be used in sensitive applications like the chemical industry.
The Solid Carbon By-product
The lack of CO2 emissions is a major benefit, but it creates a new challenge: what to do with the massive quantities of solid carbon. This carbon must be either sequestered permanently or utilized in other material production to maintain the process's low-carbon footprint.
Commercial Scalability
While steam reforming is a mature, state-of-the-art technology, methane pyrolysis has not yet been commercialized on a large scale. Significant engineering challenges remain to make it an economically viable and widespread alternative.
Making the Right Choice for Your Goal
Deciding which hydrogen production method to focus on depends entirely on your strategic priorities.
- If your primary focus is minimizing direct CO2 emissions: Pyrolysis is a powerful alternative to steam reforming, as it captures the carbon in a solid, manageable form.
- If your primary focus is energy efficiency: Pyrolysis holds a significant advantage over the vast electrical demands of green hydrogen electrolysis.
- If your primary focus is immediate, large-scale deployment: Steam reforming remains the only commercially proven and widely available technology today.
Ultimately, methane pyrolysis provides a pragmatic pathway to decarbonize hydrogen production without requiring the massive renewable energy infrastructure needed for green hydrogen.
Summary Table:
| Aspect | Methane Pyrolysis | Steam Methane Reforming (SMR) | Green Electrolysis |
|---|---|---|---|
| Primary Feedstock | Natural Gas (Methane) | Natural Gas (Methane) | Water |
| Key By-product | Solid Carbon (Carbon Black) | Carbon Dioxide (CO2) | Oxygen |
| Direct CO2 Emissions | Zero | High | Zero |
| Energy Intensity | Lower | Moderate | Very High |
| Commercial Status | Emerging / Pilot Scale | Mature / Widespread | Growing / Scaling |
Ready to explore hydrogen solutions for your laboratory or pilot project?
At KINTEK, we specialize in providing advanced lab equipment for energy research and material science. Whether you are developing pyrolysis processes, analyzing carbon by-products, or testing hydrogen purity, our expertise and reliable consumables can support your innovation.
Contact our experts today to discuss how KINTEK can equip your lab for the future of clean energy.
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Vertical Laboratory Tube Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are the advantages of a tube furnace? Achieve Superior Thermal Control and Purity
- What is the pressure on a tube furnace? Essential Safety Limits for Your Lab
- Why is an Alumina Ceramic Tube Support Necessary for 1100°C Experiments? Ensure Data Accuracy and Chemical Inertness
- How do you clean a tube furnace tube? A Step-by-Step Guide to Safe and Effective Cleaning
- What are the advantages of using an alumina liner in a tube furnace for biomass combustion corrosion simulations?



















