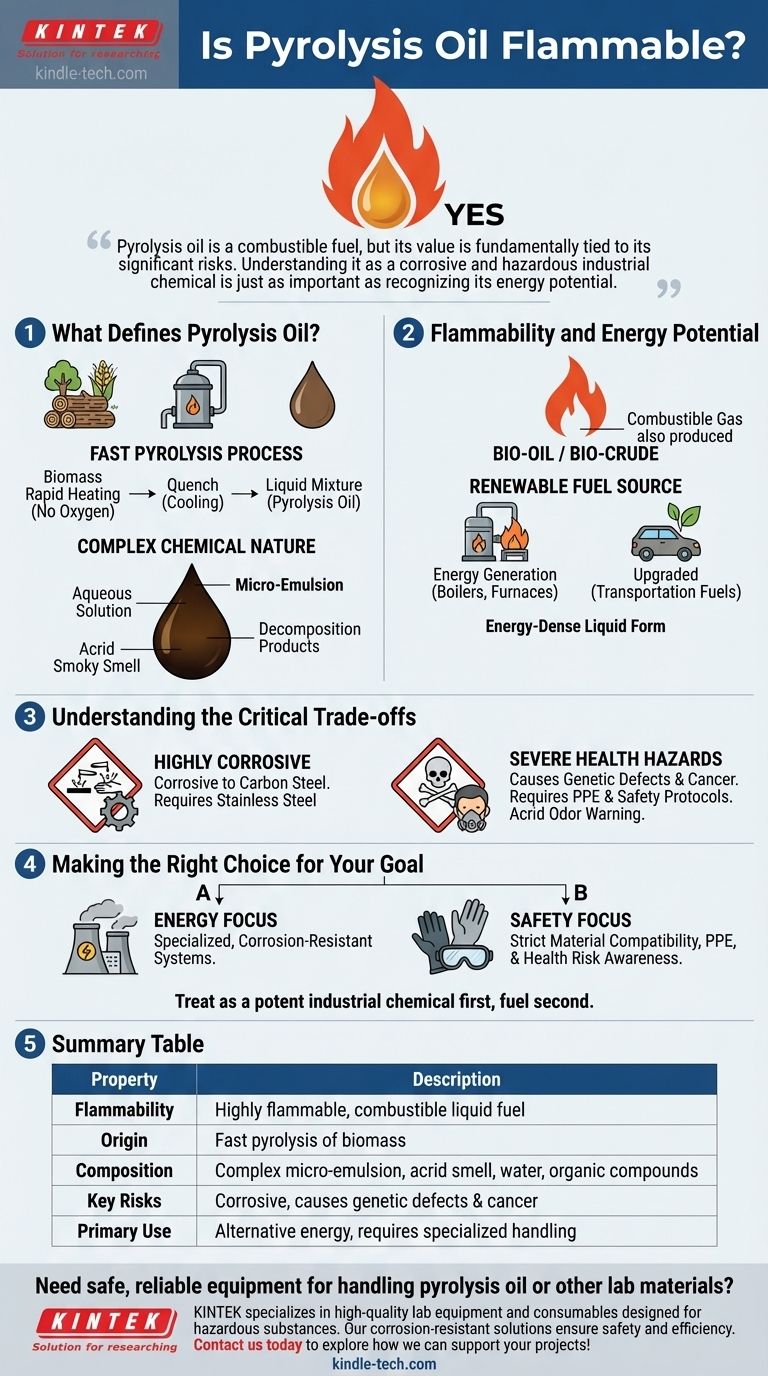
Yes, pyrolysis oil is flammable. It is a combustible liquid fuel created by the thermal decomposition of biomass. Its flammability is the primary reason it is considered a promising alternative energy source, though its complex nature requires specialized handling and safety precautions.
Pyrolysis oil is a combustible fuel, but its value is fundamentally tied to its significant risks. Understanding it as a corrosive and hazardous industrial chemical is just as important as recognizing its energy potential.

What Defines Pyrolysis Oil?
To grasp its properties, we must first understand its origin and composition. Pyrolysis oil is not a simple substance; it's a complex mixture that reflects the biomass from which it was derived.
The Fast Pyrolysis Process
Pyrolysis oil is created through a process called fast pyrolysis. This involves rapidly heating biomass (like wood or agricultural waste) in an atmosphere with little to no oxygen.
The material is then quenched, or cooled down very quickly. This rapid cycle "freezes" the chemical reactions, capturing a liquid mixture of intermediate decomposition products that would otherwise form char or gas.
A Complex Chemical Nature
The resulting liquid is a dark brown micro-emulsion. It consists of an aqueous solution containing decomposition products from cellulose, which stabilizes larger molecules derived from lignin.
This unique composition gives the oil a distinctive and acrid smoky smell and dictates its challenging properties.
Flammability and Energy Potential
The core value of pyrolysis oil lies in its ability to burn. The same process that creates the liquid oil also produces a combustible gas, which is often recycled to provide the energy needed to heat the pyrolysis reactor, making the process more efficient.
A Renewable Fuel Source
Because it is flammable, pyrolysis oil is often referred to as bio-oil or bio-crude. It can be used in boilers and furnaces for heat generation or upgraded to produce more refined liquid transportation fuels.
Its elemental composition is similar to the original biomass, but it exists in a much more energy-dense liquid form, making it easier to transport and store than raw solid biomass.
Understanding the Critical Trade-offs
While its flammability makes it a valuable fuel, pyrolysis oil is accompanied by significant risks that cannot be overlooked. It is fundamentally different from conventional petroleum fuels.
Highly Corrosive
The oil is known to be corrosive to common metals like carbon steel. This means any equipment, piping, or storage tanks used to handle it must be made from resistant materials, such as stainless steel, which increases infrastructure costs.
Severe Health Hazards
Direct exposure to pyrolysis oil is extremely dangerous. The references clearly state it can cause genetic defects and cancer.
Its acrid odor is a clear warning sign. Proper handling requires comprehensive personal protective equipment (PPE) and stringent safety protocols to prevent any skin contact or inhalation of vapors.
Making the Right Choice for Your Goal
Your approach to pyrolysis oil should be dictated by your primary objective, whether that's harnessing its energy or simply managing it safely.
- If your primary focus is energy generation: Treat it as a valuable but challenging fuel that requires specialized, corrosion-resistant combustion systems to unlock its potential.
- If your primary focus is safety and handling: Prioritize material compatibility for storage and enforce strict use of PPE, recognizing its severe long-term health risks.
Ultimately, pyrolysis oil should be treated as a potent industrial chemical first and a fuel second.
Summary Table:
| Property | Description |
|---|---|
| Flammability | Highly flammable, combustible liquid fuel (bio-oil/bio-crude) |
| Origin | Produced via fast pyrolysis of biomass (wood, agricultural waste) |
| Composition | Complex micro-emulsion with acrid smell; contains water and decomposed organic compounds |
| Key Risks | Corrosive to carbon steel, causes genetic defects and cancer upon exposure |
| Primary Use | Alternative energy source for boilers/furnaces; requires specialized handling |
Need safe, reliable equipment for handling pyrolysis oil or other lab materials? KINTEK specializes in high-quality lab equipment and consumables designed for hazardous substances. Our corrosion-resistant solutions ensure safety and efficiency for your laboratory's unique needs. Contact us today to explore how we can support your projects with dependable, tailored equipment!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Lab-Scale Vacuum Induction Melting Furnace
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
People Also Ask
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy





