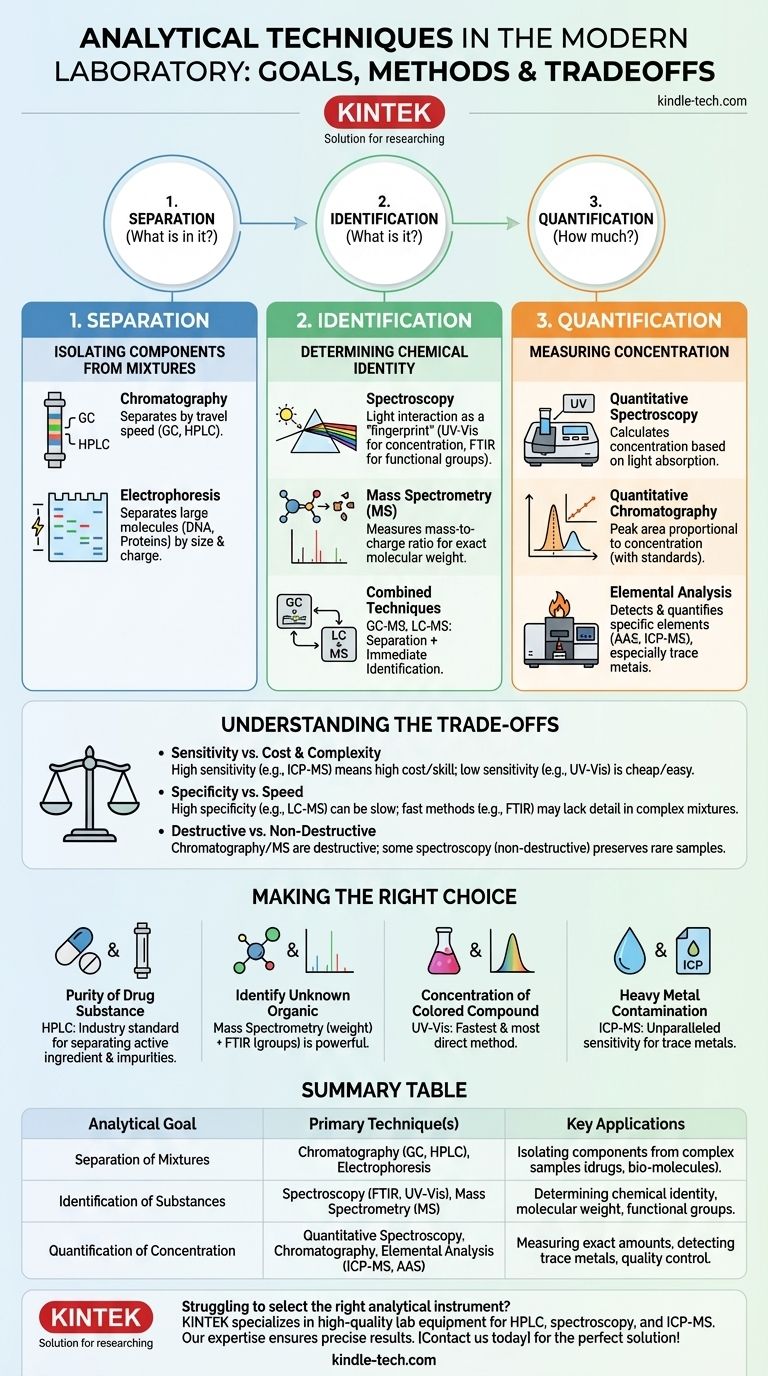
In a modern laboratory, analytical work relies on a core set of techniques designed to separate, identify, and quantify chemical substances. The most common categories of these techniques are chromatography for separation, spectroscopy for identification based on light interaction, and mass spectrometry for determining a molecule's exact mass. The specific instrument chosen depends entirely on the question you need to answer about your sample.
The challenge is not memorizing a list of instruments, but understanding the fundamental question each one is designed to answer. Analytical science boils down to three primary goals: separating mixtures, identifying what's present, and quantifying how much is there.

The Three Core Questions in Analysis
Every analytical task begins with a question. Is my sample a pure substance or a mixture? What is the chemical identity of the substance? What is its concentration? Your choice of instrument is a direct response to one of these questions.
Question 1: "What is in my complex sample?" (Separation)
Before you can identify or quantify anything, you often need to isolate it from a complex mixture. This is the domain of separation science.
Chromatography
Chromatography is a technique that separates components of a mixture by passing it through a medium where each component travels at a different speed. Think of it as a race where different molecules are the runners.
The two most common forms are Gas Chromatography (GC), for volatile substances, and High-Performance Liquid Chromatography (HPLC), for soluble substances.
Electrophoresis
This technique is fundamental in biology and biochemistry. It separates large molecules like DNA, RNA, and proteins based on their size and electrical charge by moving them through a gel matrix with an electric field.
Question 2: "What is this substance?" (Identification)
Once a substance is isolated, or if you start with a pure sample, the next step is to determine its chemical identity.
Spectroscopy
Spectroscopy studies how matter interacts with electromagnetic radiation (like UV, visible, or infrared light). Different molecules absorb and transmit light in unique ways, creating a "fingerprint."
UV-Visible (UV-Vis) Spectroscopy is often used to measure concentration, while Fourier-Transform Infrared (FTIR) Spectroscopy is excellent for identifying the specific chemical bonds (functional groups) within a molecule.
Mass Spectrometry (MS)
Mass Spectrometry is one of the most powerful analytical techniques available. It measures the precise mass-to-charge ratio of ionized molecules. This provides the exact molecular weight, which is a critical piece of information for identifying a compound.
Often, chromatography and mass spectrometry are combined (GC-MS or LC-MS) to first separate a mixture and then immediately identify each component as it emerges.
Question 3: "How much of it is there?" (Quantification)
Quantification is about determining the concentration of a substance. Many identification techniques can also be used for quantification.
Quantitative Spectroscopy
By measuring how much light a sample absorbs at a specific wavelength, UV-Vis spectroscopy can be used to calculate the concentration of a known substance in a solution. This is a foundational method in many quality control labs.
Quantitative Chromatography
When using HPLC or GC, the area under a component's peak is proportional to its concentration. By running known standards, you can build a calibration curve to accurately quantify a substance even in a complex mixture.
Elemental Analysis
Sometimes, the question is not about molecules but about atoms. Atomic Absorption Spectroscopy (AAS) and Inductively Coupled Plasma Mass Spectrometry (ICP-MS) are incredibly sensitive methods used to detect and quantify specific elements, especially trace metals in environmental or biological samples.
Understanding the Trade-offs
No single instrument is perfect for every task. Choosing the right one involves balancing competing factors.
Sensitivity vs. Cost and Complexity
An instrument like ICP-MS can detect elements at parts-per-trillion levels, but it is extremely expensive and requires a highly skilled operator. A UV-Vis spectrophotometer, on the other hand, is far less sensitive but is inexpensive, robust, and easy to use.
Specificity vs. Speed
FTIR spectroscopy can give you a result in under a minute, but if your sample is a complex mixture, the resulting spectrum can be difficult to interpret. LC-MS provides extremely high specificity but requires more extensive sample preparation and longer run times.
Destructive vs. Non-Destructive Analysis
Most chromatographic and mass spectrometry techniques are destructive, meaning the sample is consumed during the analysis. Some spectroscopic methods, however, can be non-destructive, which is critical if your sample is rare or precious.
Making the Right Choice for Your Goal
Your analytical strategy should be dictated by your ultimate objective. Use the instrument that answers your specific question most efficiently.
- If your primary focus is to determine the purity of a drug substance: HPLC is the industry standard for separating the active ingredient from any impurities.
- If your primary focus is to identify an unknown organic compound: A combination of Mass Spectrometry (for molecular weight) and FTIR (for functional groups) is a powerful approach.
- If your primary focus is to measure the concentration of a known colored compound in water: UV-Vis spectroscopy is the fastest and most direct method.
- If your primary focus is to check for heavy metal contamination in drinking water: ICP-MS provides the unparalleled sensitivity required for regulatory compliance.
Choosing the right analytical tool is the first step in transforming a sample from an unknown into a source of actionable knowledge.
Summary Table:
| Analytical Goal | Primary Technique(s) | Key Applications |
|---|---|---|
| Separation of Mixtures | Chromatography (GC, HPLC), Electrophoresis | Isolating components from complex samples like drugs or biological molecules |
| Identification of Substances | Spectroscopy (FTIR, UV-Vis), Mass Spectrometry (MS) | Determining chemical identity, molecular weight, and functional groups |
| Quantification of Concentration | Quantitative Spectroscopy, Chromatography, Elemental Analysis (ICP-MS, AAS) | Measuring exact amounts, detecting trace metals, quality control |
Struggling to select the right analytical instrument for your specific lab challenges? KINTEK specializes in providing high-quality lab equipment and consumables tailored to your needs—whether you're separating complex mixtures with HPLC, identifying compounds via spectroscopy, or quantifying trace elements with ICP-MS. Our expertise ensures you get precise, reliable results. Contact us today to find the perfect solution for your laboratory!
Visual Guide
Related Products
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Custom PTFE Teflon Parts Manufacturer for Centrifuge Tube Racks
People Also Ask
- What are the disadvantages of XRF? Understanding Its Limits for Accurate Elemental Analysis
- What are 3 types of biomass? A Guide to Wood, Waste, and Biofuels for Energy
- What temperature do you braze stainless steel? Master the Key Factors for a Perfect Joint
- What precautions should be taken during a heat treatment experiment? Essential Safety Protocols for Your Lab
- How do you increase filter press efficiency? Optimize Your Dewatering System for Maximum Output
- What are the functions of laboratory shakers and centrifuges in phosphorus extraction? Optimize Sample Purification
- What is the process of thin film production? A Guide to Atomic-Level Material Engineering
- Does water bath evaporate? Yes, and here’s how to control it effectively.










