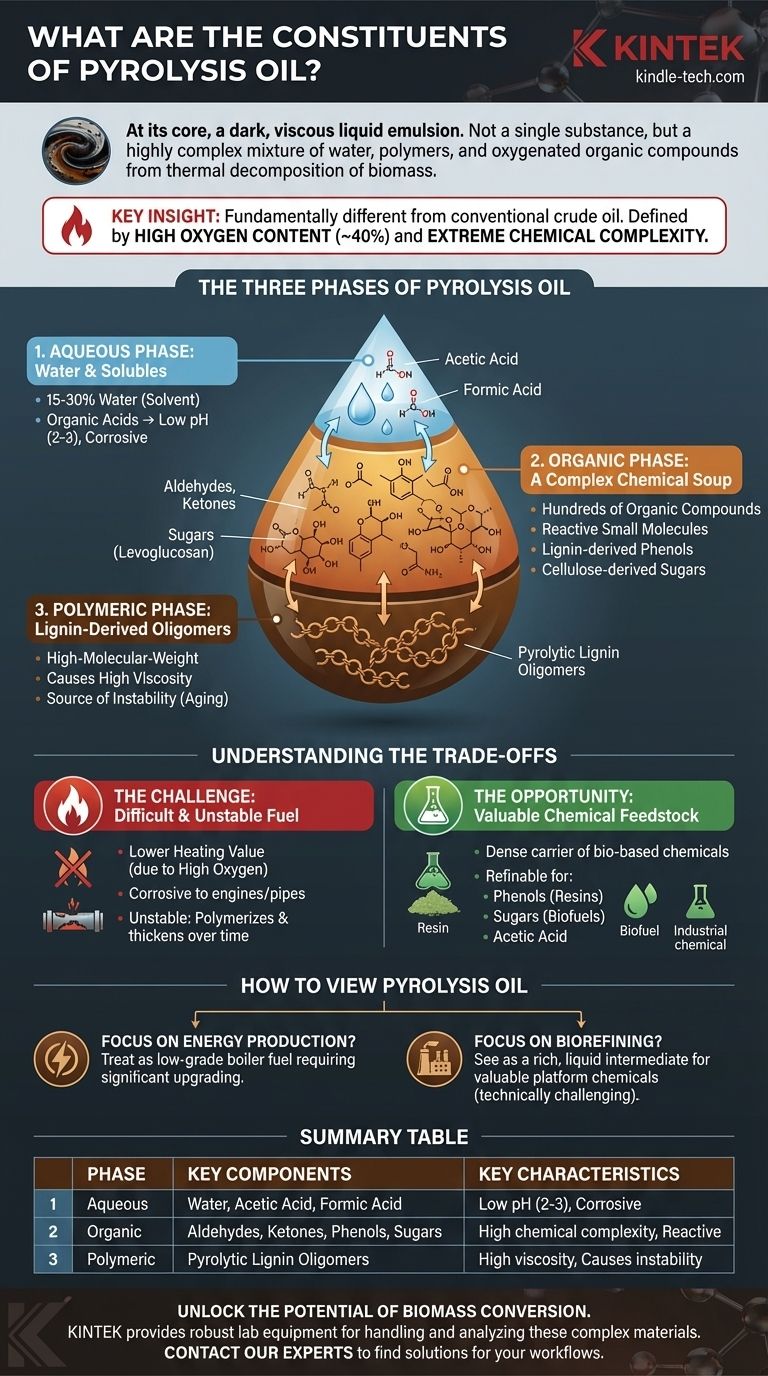
At its core, pyrolysis oil, often called bio-oil or bio-crude, is a dark, viscous liquid emulsion. It is not a single substance but a highly complex mixture of water, polymers, and hundreds of different oxygenated organic compounds produced from the thermal decomposition of biomass in the absence of oxygen.
The most critical thing to understand is that pyrolysis oil is fundamentally different from conventional crude oil. Its high oxygen content (up to 40%) and extreme chemical complexity are its defining characteristics, dictating both its challenges as a fuel and its potential as a chemical feedstock.

The Three Phases of Pyrolysis Oil
Pyrolysis oil is best understood not as a true solution but as a micro-emulsion consisting of three interconnected phases. The proportion of these phases depends heavily on the original biomass feedstock and the pyrolysis process conditions.
The Aqueous Phase: Water and Solubles
The oil contains a significant amount of water, typically ranging from 15-30% by weight. This is not just an impurity; it is an integral part of the liquid, created during the pyrolysis reaction and acting as a solvent.
This aqueous phase holds the water-soluble, low-molecular-weight compounds. This includes organic acids like acetic acid and formic acid, which are responsible for the oil's low pH (typically 2-3) and its corrosive nature.
The Organic Phase: A Complex Chemical Soup
This is the heart of pyrolysis oil's complexity, containing hundreds of distinct organic compounds derived from the breakdown of cellulose, hemicellulose, and lignin.
These compounds can be grouped into several families:
- Acids, Aldehydes, and Ketones: Small, reactive molecules like formaldehyde, hydroxyacetone, and furfural.
- Phenols: A wide range of phenolic compounds derived from the breakdown of lignin in the biomass.
- Sugars: Anhydrosugars like levoglucosan, formed from the decomposition of cellulose.
The Polymeric Phase: Lignin-Derived Oligomers
This phase consists of larger, water-insoluble molecules, often referred to as pyrolytic lignin. These are high-molecular-weight oligomers primarily derived from the lignin in the original feedstock.
These large molecules are responsible for the oil's high viscosity and its tendency to thicken over time.
Understanding the Trade-offs of Its Composition
The unique chemical makeup of pyrolysis oil creates a distinct set of advantages and disadvantages. Acknowledging these is crucial for any practical application.
The Challenge: A Difficult and Unstable Fuel
The very properties that define pyrolysis oil make it a poor direct substitute for conventional fuels like diesel or heating oil.
Its high oxygen content results in a lower heating value, meaning you get less energy per kilogram compared to fossil fuels. The presence of organic acids makes it corrosive to standard pipes and engines, requiring specialized materials. Finally, the reactive aldehydes and phenols cause the oil to be unstable, aging over time by polymerizing, which increases its viscosity until it can become a semi-solid.
The Opportunity: A Valuable Chemical Feedstock
The complexity of pyrolysis oil can also be seen as its greatest strength. It is a dense liquid carrier of valuable, bio-based chemicals.
Instead of being burned for its low-grade energy, the oil can be refined. The phenols can be extracted to produce bio-based resins and adhesives, the sugars can be fermented into biofuels or other chemicals, and the acetic acid can be recovered for industrial use.
How to View Pyrolysis Oil
Your perspective on pyrolysis oil should be guided by your ultimate goal. It is not a one-size-fits-all commodity.
- If your primary focus is energy production: You must treat it as a low-grade boiler fuel that requires significant upgrading or specialized combustion systems to manage its high water content, corrosivity, and instability.
- If your primary focus is biorefining: You should see it as a rich, liquid intermediate for producing valuable platform chemicals, but be prepared for the significant technical challenges of separating and purifying these compounds from the complex mixture.
Ultimately, understanding its composition as a reactive, oxygen-rich emulsion—not a simple oil—is the key to unlocking its true potential.
Summary Table:
| Phase | Key Components | Key Characteristics |
|---|---|---|
| Aqueous Phase | Water, Acetic Acid, Formic Acid | Low pH (2-3), Corrosive, 15-30% of oil |
| Organic Phase | Aldehydes, Ketones, Phenols, Sugars | High chemical complexity, Reactive |
| Polymeric Phase | Pyrolytic Lignin Oligomers | High viscosity, Causes instability |
Unlock the potential of biomass conversion in your lab. Pyrolysis oil presents unique challenges and opportunities, whether for energy research or chemical extraction. KINTEK specializes in providing the robust lab equipment and consumables needed to safely handle, analyze, and process these complex materials. Contact our experts today to find the right solutions for your pyrolysis and biorefining workflows.
Contact Us for Your Lab Equipment Needs
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Large Vertical Graphite Vacuum Graphitization Furnace
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
People Also Ask
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time










