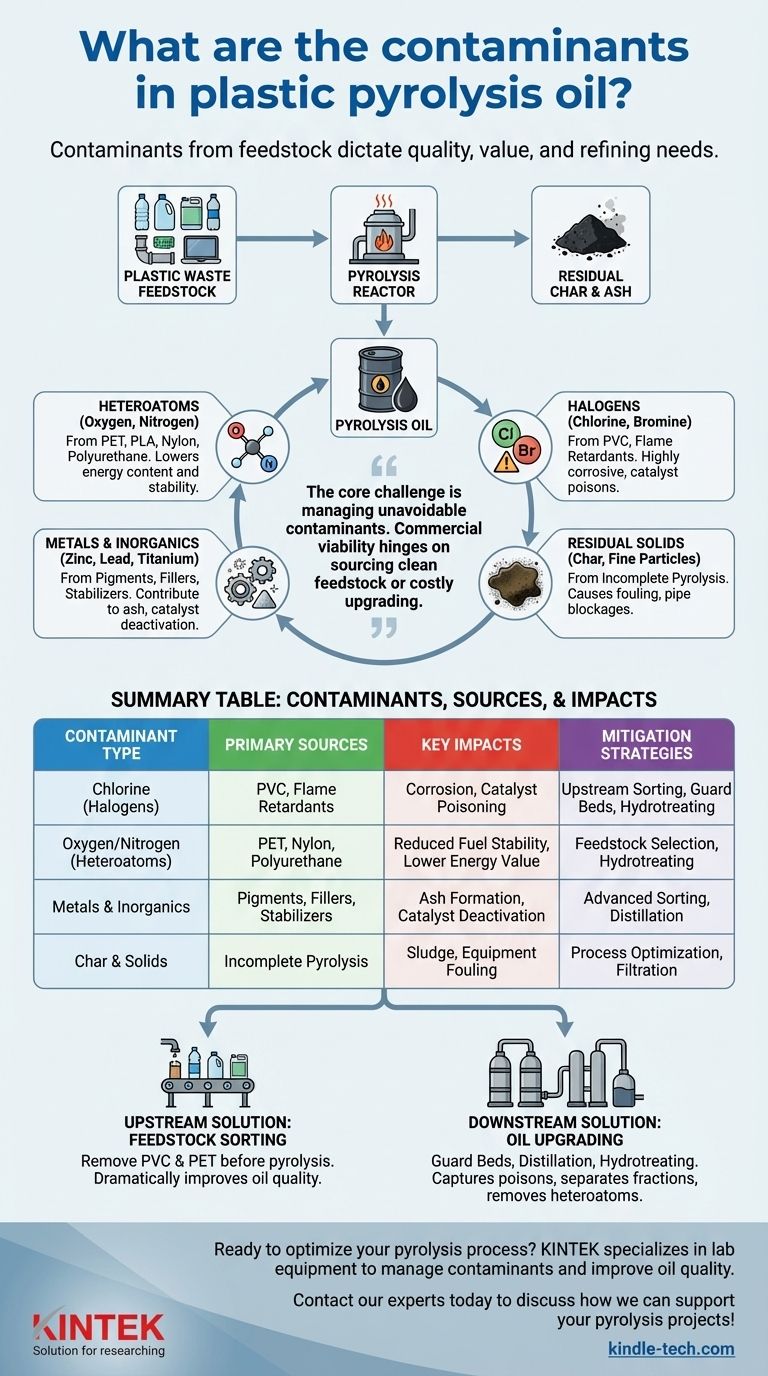
The primary contaminants in plastic pyrolysis oil are heteroatoms, halogens, metals, and inorganic solids that originate directly from the composition of the plastic waste feedstock. These impurities include chlorine from PVC, oxygen from PET, nitrogen from polyamides, bromine from flame retardants, and various metals used as pigments and stabilizers. The presence and concentration of these contaminants dictate the oil's quality, value, and suitability for further refining.
The core challenge of plastic pyrolysis is not simply converting plastic to oil, but managing the unavoidable contaminants inherited from the original waste. The commercial viability of any pyrolysis operation hinges on its ability to either source clean feedstock or implement costly upgrading steps to purify the final product.

The Origin of Contaminants: From Waste to Oil
The chemical principle is simple: what you put into the reactor is what you get out. Unlike crude oil, which has been naturally refined over millennia, plastic waste is a complex mixture of polymers, additives, and non-plastic materials.
Heteroatoms: The Non-Hydrocarbon Elements
Ideal fuels are pure hydrocarbons (hydrogen and carbon). Many common plastics, however, contain other elements called heteroatoms, which become integrated into the oil's molecular structure.
The most significant are oxygen, from Polyethylene terephthalate (PET) and Polylactic acid (PLA), and nitrogen, from polyamides (Nylon) and polyurethane. These elements result in oxygenated and nitrogenated compounds that lower the oil's energy content and stability.
Halogens: Chlorine and Bromine
Halogens are particularly problematic contaminants. Chlorine is the most notorious, primarily coming from Polyvinyl chloride (PVC). During pyrolysis, it forms highly corrosive hydrochloric acid (HCl) gas, which can severely damage equipment and poison downstream refining catalysts.
Bromine originates from brominated flame retardants (BFRs) commonly used in electronics plastics (e.g., ABS) and construction materials. Like chlorine, it is highly corrosive and a catalyst poison.
Metals and Inorganics
Plastics contain a wide array of inorganic additives. These include pigments (e.g., titanium dioxide for white), fillers (e.g., calcium carbonate to add bulk), and stabilizers that may contain zinc, lead, and cadmium.
During pyrolysis, these materials largely concentrate in the solid char byproduct. However, some can be carried over into the oil as fine particulates or volatile organometallic compounds, contributing to ash formation and acting as catalyst poisons.
Residual Char and Ash
Not all organic material vaporizes during pyrolysis. A solid residue known as char is always produced. Fine particles of this char can become entrained in the oil vapors and condense with the liquid, creating a physical sludge that can block pipes and foul equipment.
Understanding the Impact of Contaminants
These contaminants are not minor impurities; they fundamentally limit the pyrolysis oil's use and create significant operational hazards.
Corrosion and Equipment Damage
Hydrochloric acid (from PVC) and hydrobromic acid (from BFRs) are extremely corrosive to steel, especially at the high temperatures of a pyrolysis system. This necessitates the use of expensive alloys and robust maintenance schedules, increasing capital and operational costs.
Catalyst Poisoning
Perhaps the single greatest barrier to using pyrolysis oil is catalyst poisoning. Traditional oil refineries use highly sensitive catalysts for processes like fluid catalytic cracking (FCC) and hydrotreating.
Elements like chlorine, sulfur, nitrogen, lead, and zinc permanently deactivate these catalysts, even at parts-per-million levels. This makes co-processing pyrolysis oil with conventional crude oil impossible without extensive and costly pre-treatment.
Reduced Fuel Quality and Stability
Oxygenates reduce the oil's heating value, meaning more of it must be burned to produce the same amount of energy. Furthermore, compounds containing oxygen and nitrogen are often reactive, causing the oil to slowly degrade, polymerize, and form gums and sediments during storage.
Common Pitfalls and Mitigation Strategies
Addressing contaminants requires a systems-level approach, involving trade-offs between cost, complexity, and final product quality.
The Myth of a "Clean" Feedstock
Even a seemingly clean stream of a single plastic type, like polypropylene, will still contain proprietary blends of stabilizers, pigments, and processing aids. Assuming any real-world plastic waste is "pure" is a common and costly mistake.
Upstream Solution: Feedstock Sorting
The most effective strategy is to remove problematic plastics before they enter the reactor. Advanced sorting technologies can identify and separate PVC and PET, which are the sources of the most troublesome chlorine and oxygen contaminants. This adds cost and complexity but dramatically improves the quality of the resulting oil.
Downstream Solution: Oil Upgrading
After production, the pyrolysis oil must be "upgraded" to meet refinery specifications. This is a multi-step process that can include:
- Guard Beds: Using adsorbents to capture specific poisons like chlorine.
- Distillation: Separating the oil into different fractions, similar to a traditional refinery.
- Hydrotreating: A high-pressure, high-temperature process that uses hydrogen and a catalyst to remove heteroatoms (Cl, N, O, S) and saturate unstable molecules. This is effective but energy-intensive and expensive.
Making the Right Choice for Your Goal
Your strategy for managing contaminants must align with your end-product objective.
- If your primary focus is producing a refinery-ready feedstock: Your absolute priority must be aggressive pre-treatment to remove chlorine (PVC) and comprehensive downstream upgrading, especially hydrotreating.
- If your primary focus is creating a lower-grade fuel for furnaces or boilers: You can tolerate higher levels of some contaminants, but chlorine and metals must still be minimized to prevent corrosion and operational issues.
- If your primary focus is evaluating the economic viability of a pyrolysis project: The cost of contaminant removal—both upstream sorting and downstream upgrading—must be a central line item in your financial model, as it often determines profitability.
Understanding and managing these contaminants is the central engineering challenge that separates a theoretical process from a commercially successful circular economy solution.
Summary Table:
| Contaminant Type | Primary Sources | Key Impacts |
|---|---|---|
| Chlorine (Halogens) | PVC, flame retardants | Corrosion, catalyst poisoning |
| Oxygen/Nitrogen (Heteroatoms) | PET, Nylon, polyurethane | Reduced fuel stability, lower energy value |
| Metals & Inorganics | Pigments, fillers, stabilizers | Ash formation, catalyst deactivation |
| Char & Solids | Incomplete pyrolysis | Sludge, equipment fouling |
Ready to optimize your pyrolysis process with reliable lab equipment? KINTEK specializes in furnaces, reactors, and analytical tools that help you manage contaminants and improve oil quality. Whether you're scaling up R&D or ensuring operational efficiency, our solutions are designed for your laboratory's needs. Contact our experts today to discuss how we can support your pyrolysis projects!
Visual Guide
Related Products
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
People Also Ask
- What are the disadvantages of sieve machine? Key Limitations in Particle Size Analysis
- Why is powder classification using standard sieves essential for SHS reactions? Unlock Superior Nitriding Results
- How do high-precision sieving systems benefit zeolite preparation? Maximize Adsorption for Wastewater Treatment
- Why is a laboratory sieving system required for bentonite in coatings? Ensure Flawless Surface Performance
- What is the significance of using a fine sieving system for catalyst particles? Optimize Size for Maximum Reactivity