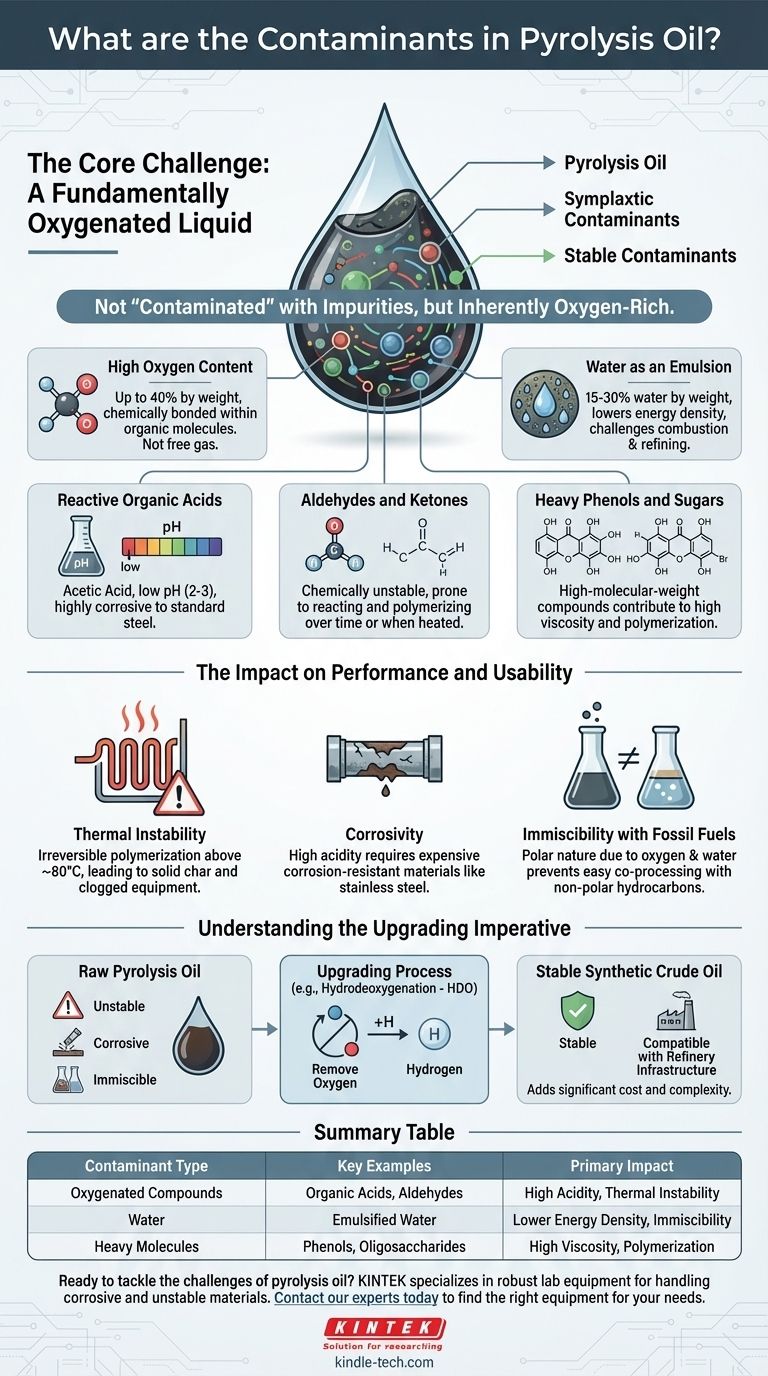
The primary contaminants in pyrolysis oil are oxygen, water, and a wide range of reactive oxygenated compounds. Unlike conventional crude oil, which is composed almost entirely of hydrocarbons, pyrolysis oil's "contamination" is its inherent chemical nature. This high oxygen content is the root cause of its most challenging properties, including high acidity, thermal instability, and immiscibility with fossil fuels.
The term "contaminant" can be misleading. Pyrolysis oil is not contaminated in the way crude oil is with sulfur. Instead, its fundamental composition—a complex emulsion of water and oxygen-rich organic molecules—is the primary barrier to its use as a direct, drop-in fuel.

The Core Challenge: A Fundamentally Oxygenated Liquid
The issues with pyrolysis oil stem directly from the biomass it comes from. Biomass is rich in oxygen, and the pyrolysis process preserves much of that oxygen in the final liquid product.
High Oxygen Content
Pyrolysis oil can contain up to 40% oxygen by weight. This oxygen is not free gas but is chemically bonded within the organic molecules, forming a complex mixture that is fundamentally different from hydrocarbons.
Water as an Emulsion
The oil is also an emulsion containing a significant amount of water, often 15-30% by weight. This water is finely dispersed and intimately mixed, lowering the oil's energy density and creating challenges for combustion and refining.
Reactive Organic Acids
A significant portion of the oxygen exists in the form of organic acids, most notably acetic acid. These acids give the oil a very low pH (typically 2-3), making it highly corrosive to standard carbon steel equipment like pipes, pumps, and storage tanks.
Aldehydes and Ketones
Compounds like formaldehyde are also present. These, along with other reactive species, make the oil chemically unstable. They are prone to reacting with each other over time or when heated.
Heavy Phenols and Sugars
The oil also contains larger, more complex molecules like phenols and oligosaccharides (sugars). These high-molecular-weight compounds contribute to the oil's high viscosity and its tendency to polymerize.
The Impact on Performance and Usability
These inherent chemical properties create significant practical challenges that prevent pyrolysis oil from being a simple replacement for petroleum products.
Thermal Instability
When heated above approximately 80°C, the reactive oxygenated compounds begin to polymerize. This process irreversibly thickens the oil, eventually turning it into a solid char or coke, which can clog fuel lines and foul processing equipment.
Corrosivity
The high acidity requires that all infrastructure handling pyrolysis oil—from storage tanks to engine components—be constructed from expensive, corrosion-resistant materials like stainless steel.
Immiscibility with Fossil Fuels
Pyrolysis oil does not mix with non-polar hydrocarbon fuels like gasoline or diesel. This is because its high oxygen and water content make it a polar liquid, similar to water itself. This prevents it from being easily co-processed in traditional oil refineries.
Understanding the Upgrading Imperative
It is crucial to understand that these "contaminants" are not accidental impurities but are an intrinsic feature of raw pyrolysis oil.
A Feature, Not a Bug
The oxygenated composition is a direct result of the low-temperature thermal decomposition of biomass. Producing a low-oxygen oil would require a completely different process, such as high-pressure hydrotreating.
The Need for Upgrading
Because of its instability, corrosivity, and immiscibility, raw pyrolysis oil cannot be used as a "drop-in" fuel. It must first undergo an upgrading process, most commonly hydrodeoxygenation (HDO), to remove oxygen by reacting it with hydrogen.
This upgrading step converts the oxygenated molecules into stable hydrocarbons, producing a synthetic crude oil that is compatible with existing refinery infrastructure. However, this process adds significant cost and complexity.
Making the Right Choice for Your Goal
Your strategy for dealing with pyrolysis oil depends entirely on your end objective.
- If your primary focus is producing transport fuels: You must plan for a robust and costly upgrading process to remove oxygen, stabilize the oil, and make it compatible with conventional refineries.
- If your primary focus is stationary heat or power: You may be able to use the raw oil directly in specially designed boilers or turbines built with corrosion-resistant materials and engineered to handle its unique properties.
- If your primary focus is extracting valuable chemicals: View the oxygenated compounds, like phenols, not as contaminants but as products. Your goal will be to develop separation and purification technologies to isolate these high-value chemicals.
Understanding these inherent properties is the first step toward effectively upgrading, handling, or extracting value from this complex renewable liquid.
Summary Table:
| Contaminant Type | Key Examples | Primary Impact |
|---|---|---|
| Oxygenated Compounds | Organic Acids (Acetic Acid), Aldehydes (Formaldehyde) | High Acidity (Corrosivity), Thermal Instability |
| Water | Emulsified Water (15-30%) | Lower Energy Density, Immiscibility with Fossil Fuels |
| Heavy Molecules | Phenols, Oligosaccharides | High Viscosity, Tendency to Polymerize |
Ready to tackle the challenges of pyrolysis oil? KINTEK specializes in providing robust lab equipment and consumables designed for handling corrosive and unstable materials. Whether you're upgrading bio-oil, testing its properties, or extracting valuable chemicals, our solutions ensure safety and accuracy. Contact our experts today to find the right equipment for your laboratory's unique needs.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
- High Performance Laboratory Freeze Dryer for Research and Development
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Automatic Lab Cold Isostatic Press CIP Machine Cold Isostatic Pressing
People Also Ask
- How does PECVD work? Enable Low-Temperature, High-Quality Thin Film Deposition
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application
- What are the different types of thin films? A Guide to Optical, Electrical, and Functional Coatings
- What is the difference between PECVD and CVD? Unlock the Right Thin-Film Deposition Method
- What are the steps of the CVD process? A Guide to Precision Thin Film Deposition


















