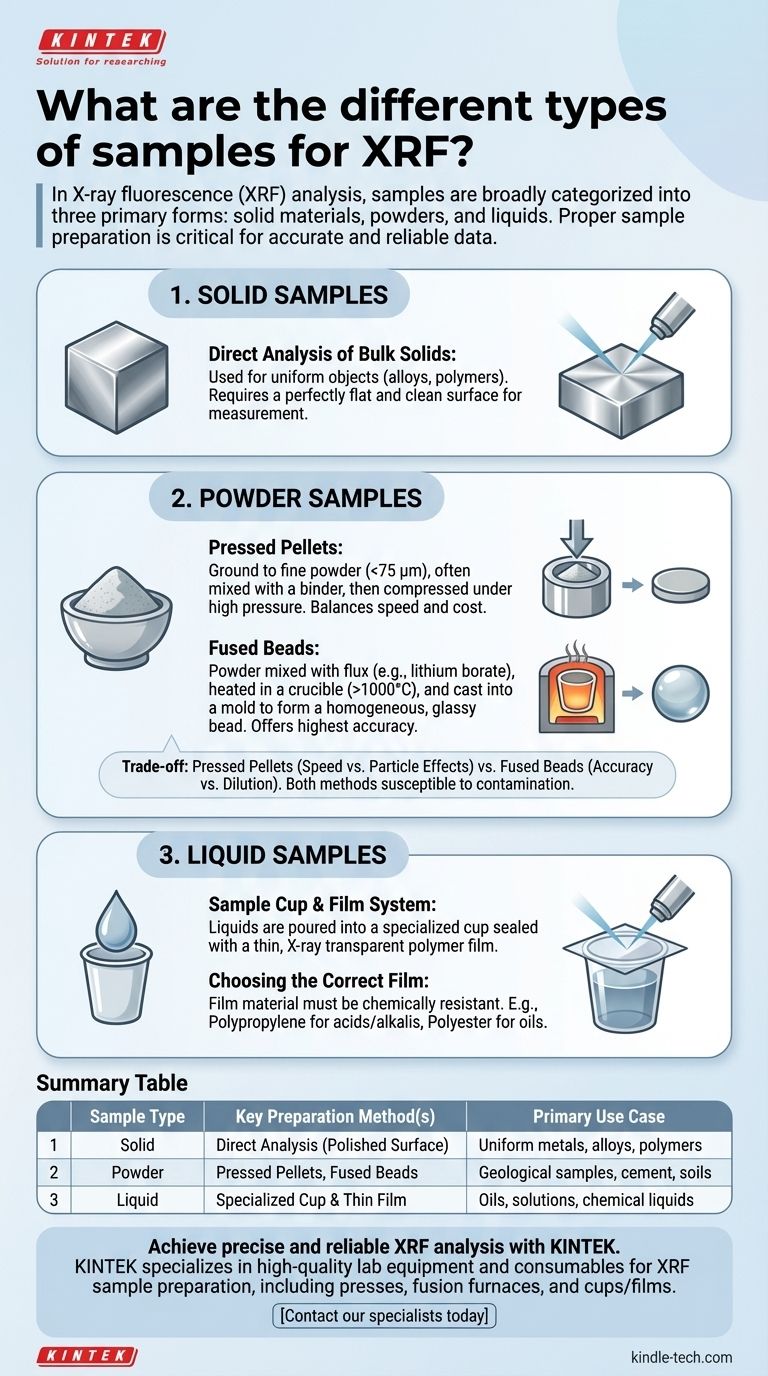
In X-ray fluorescence (XRF) analysis, samples are broadly categorized into three primary forms: solid materials, powders, and liquids. Solid samples can be analyzed directly if they have a flat surface, or they can be ground into a fine powder. These powders are then typically prepared as either pressed pellets or fused beads for analysis. Liquid samples are contained in specialized cups sealed with a thin film.
The accuracy of your XRF results is not determined by the spectrometer alone. Proper sample preparation is a critical, non-negotiable step that ensures the data you collect is both reliable and representative of the material you are analyzing.

The Foundation: Why Sample Preparation Dictates XRF Accuracy
Before examining specific sample types, it is essential to understand the physical principles that make preparation so critical. XRF is a surface-sensitive technique, and two factors—geometry and homogeneity—have an outsized impact on your results.
The Importance of a Flat, Consistent Surface
An XRF spectrometer is calibrated for a precise distance between the X-ray source, the sample, and the detector. Any variation in this distance will alter the intensity of the fluorescence signal.
An irregular or non-flat surface creates microscopic variations in that distance, causing some parts of the sample to be closer or farther from the source. This introduces significant error, skewing the final elemental concentration data.
The Goal of Homogeneity
The X-ray beam only excites a small area and depth of the sample. For the analysis to be representative of the entire material, the portion being measured must be homogeneous.
If a sample has different minerals or particle sizes, grinding it into a fine, uniform powder ensures that the small area being analyzed is a true average of the bulk material.
Preparing Solid and Powdered Samples
Solids represent the most common sample type for XRF and offer the most preparation options, each suited for different analytical goals and material types.
Direct Analysis of Bulk Solids
This method is used for uniform, solid objects like metal alloys or polymers. The primary requirement is creating a perfectly flat and clean surface for measurement.
Preparation involves mechanical processing, such as using a lathe for soft metals or a grinding/polishing tool for harder materials. The surface must then be cleaned to remove any residue or contamination.
Pressed Pellets
This is one of the most common preparation methods due to its balance of speed, cost, and quality. It is ideal for geological samples, cement, and other materials that can be pulverized.
The sample is ground to a fine powder (typically less than 75 micrometers) and often mixed with a binding agent like wax or cellulose. This powder is then placed in a die and compressed under high pressure to form a stable, flat pellet.
Fused Beads
This method offers the highest level of accuracy by creating a perfectly homogeneous, glassy solid. It completely eliminates errors from particle size and mineralogical effects.
The powdered sample is mixed with a flux (such as a lithium borate) and heated in a crucible to over 1000°C. The molten mixture is then cast into a mold to cool, forming a fused bead with a perfectly flat surface for analysis.
Preparing Liquid Samples
Analyzing liquids requires containing them in a way that is transparent to the X-ray beam while preventing leaks or contamination.
The Sample Cup and Film System
Liquid samples are poured into a specialized plastic sample cup. The bottom of the cup is open and must be sealed by a thin, tightly stretched polymer film.
This film serves as the window through which the X-ray beam passes. It must be strong enough to support the liquid but thin enough to allow for maximum X-ray transmission.
Choosing the Correct Film
The choice of film material is critical and depends on the chemical nature of the liquid. The film must be chemically resistant to the sample to prevent it from dissolving or swelling.
For example, polypropylene film is often used for acids and alkalis, while polyester films (like Mylar) are suitable for oils and hydrocarbon-based products.
Understanding the Trade-offs
No single preparation method is universally superior. The right choice depends on your analytical goals, the material itself, and your available resources.
Pressed Pellets: Speed vs. Particle Effects
Pressed pellets are fast and inexpensive to prepare. However, they can still be susceptible to particle size effects, where finer or heavier particles can segregate during pressing, leading to a non-uniform surface and reduced accuracy.
Fused Beads: Accuracy vs. Dilution
Fusion creates the most accurate sample by eliminating all physical matrix effects. The significant trade-off is dilution; the sample is mixed with a large amount of flux, which lowers the concentration of every element. This can make it difficult to detect and quantify trace elements.
The Universal Risk of Contamination
All preparation methods carry a risk of contamination. Grinding equipment can introduce metallic elements, binders can contain impurities, and sample cups can be a source of contamination if not handled properly. Using separate, dedicated tools for different sample types is a crucial best practice.
Making the Right Choice for Your Goal
Your analytical objective should guide your preparation strategy. Choose the method that best aligns with your need for speed, accuracy, and the nature of your sample.
- If your primary focus is maximum accuracy and eliminating matrix effects: Fused beads are the gold standard, especially for complex geological materials or when creating calibrations.
- If your primary focus is high-throughput and routine quality control: Pressed pellets provide a fast, cost-effective, and reliable method for consistent materials.
- If your primary focus is non-destructive analysis of a uniform solid: Direct analysis of a polished bulk material is the most straightforward path, provided you can prepare a perfect surface.
- If your primary focus is analyzing liquids, oils, or solutions: The liquid cup and film method is the required approach, with careful film selection being the most critical decision.
Mastering sample preparation transforms XRF from a simple measurement into a powerful, reliable analytical technique.
Summary Table:
| Sample Type | Key Preparation Method(s) | Primary Use Case |
|---|---|---|
| Solid | Direct Analysis (Polished Surface) | Uniform metals, alloys, polymers |
| Powder | Pressed Pellets, Fused Beads | Geological samples, cement, soils |
| Liquid | Specialized Cup & Thin Film | Oils, solutions, chemical liquids |
Achieve precise and reliable XRF analysis with KINTEK.
Proper sample preparation is the foundation of accurate results. Whether you are working with solid metals, powdered minerals, or liquid samples, having the right equipment and expertise is crucial. KINTEK specializes in high-quality lab equipment and consumables for all your XRF sample preparation needs, including presses for pellets, fusion furnaces for beads, and a full range of cups and films.
Let our expertise help you eliminate matrix effects and contamination risks. Contact our specialists today to discuss your specific application and ensure your lab is equipped for success.
Visual Guide
Related Products
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
People Also Ask
- What is the pellet technique in IR? Master Solid Sample Preparation for Clear Spectroscopy
- What is the advantage of KBr? Unmatched IR Transparency for Precise Spectroscopy
- How hot is a hydraulic press? Understanding the Critical Heat in Your Hydraulic System
- Why use KBr for IR? Achieve Clear, Unobstructed Spectra for Solid Samples
- How does a laboratory hydraulic press improve XRF accuracy for catalyst samples? Enhance Precision & Signal Stability



















