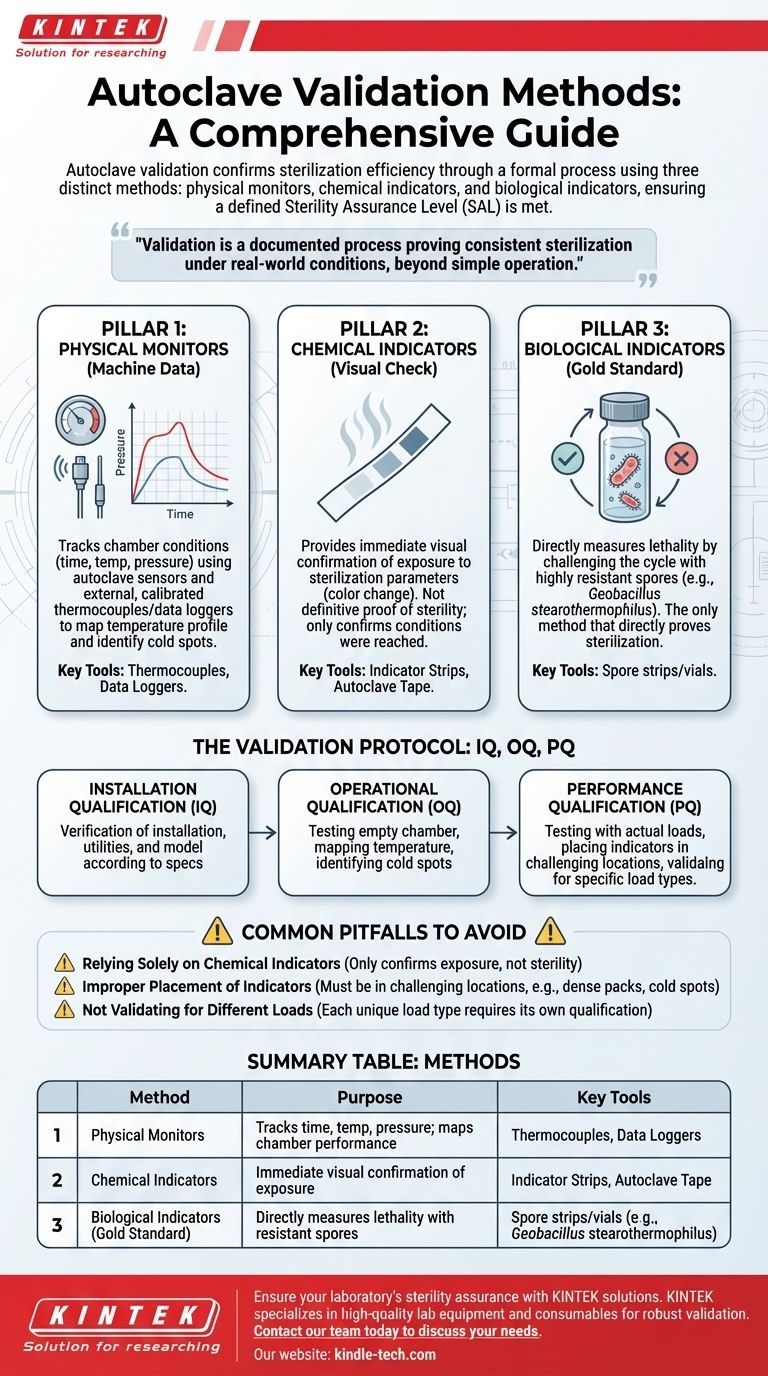
In short, autoclave validation is performed using a combination of three distinct methods: physical monitors, chemical indicators, and biological indicators. These methods are used together in a structured process to provide a comprehensive and reliable confirmation that the autoclave is achieving the required level of sterility.
Autoclave validation is not a single action, but a formal, documented process that proves the equipment consistently sterilizes its contents under real-world conditions. It moves beyond simply operating the machine to providing objective evidence that a defined Sterility Assurance Level (SAL) has been met.

The Three Pillars of Autoclave Validation
True validation relies on a multi-faceted approach. Each method provides a different piece of the puzzle, and only when used together do they offer a complete picture of the autoclave's performance.
Pillar 1: Physical Monitors (The Machine's Data)
Physical monitors are the sensors and data recorders that track the physical conditions inside the autoclave chamber during a cycle.
These include the autoclave's own temperature probes, pressure sensors, and timers. For formal validation, external, calibrated thermocouples and data loggers are placed throughout the chamber to map its temperature profile and identify any "cold spots."
This method provides a precise, time-stamped record of the cycle's physical parameters, confirming if the target temperature and pressure were reached and held for the required duration.
Pillar 2: Chemical Indicators (The Visual Check)
Chemical indicators are materials that undergo a visible change, typically color, when exposed to one or more specific sterilization parameters like temperature or steam.
These are most often seen as indicator strips or tape. They provide a quick, visual confirmation that the items inside the autoclave have been exposed to the sterilization process.
However, they are not a definitive proof of sterility. A chemical indicator changing color only confirms that a certain temperature was reached, not that it was held long enough or that steam penetrated the load effectively to kill microorganisms.
Pillar 3: Biological Indicators (The Gold Standard)
Biological indicators (BIs) are the most critical tool for validation as they directly measure the lethality of the sterilization process.
BIs consist of a standardized population of highly resistant bacterial spores, most commonly Geobacillus stearothermophilus. These spores are known to be far more difficult to kill than the common microorganisms found in a lab.
After a cycle, the BI is incubated. If no growth occurs, it provides a high degree of confidence that all other potential bioburdens in the load have been successfully destroyed. This is the only method that directly proves sterilization was achieved.
Putting It Together: The Validation Protocol
These three pillars are integrated into a formal qualification process, most often broken down into three phases known as IQ, OQ, and PQ.
Installation Qualification (IQ)
This is the initial documented check. It verifies that the autoclave is the correct model, has been installed correctly according to the manufacturer's specifications, and that all necessary utilities (power, water) are properly connected.
Operational Qualification (OQ)
In OQ, the autoclave is tested to confirm it operates correctly while empty. This phase involves mapping the chamber temperature with multiple thermocouples to ensure heat is distributed evenly and to identify the coldest locations within the chamber.
Performance Qualification (PQ)
This is the most crucial phase. The autoclave is tested with actual loads that represent the materials you will be sterilizing daily (e.g., liquids, instruments, waste bags).
During PQ, both chemical and biological indicators are strategically placed throughout the load, with a special focus on the "cold spots" identified during OQ. Successful PQ runs demonstrate that the autoclave can effectively sterilize its intended contents under real-world conditions.
Common Pitfalls to Avoid
Achieving reliable validation means being aware of common mistakes that can lead to a false sense of security.
Relying Solely on Chemical Indicators
The most frequent error is assuming that a color change on indicator tape equals sterility. It does not. Chemical indicators only confirm exposure to heat and should be used for routine monitoring, not as the sole basis for validation.
Improper Placement of Indicators
Placing BIs in an easily accessible, open area of the chamber is not a valid test. They must be placed in the most challenging locations for steam to penetrate: inside dense packs, within liquid containers, or in the identified cold spots.
Not Validating for Different Loads
A sterilization cycle that works for a tray of glassware may fail for a large flask of liquid media. Each unique load type and configuration requires its own performance qualification to ensure it can be reliably sterilized.
Making the Right Choice for Your Goal
Your approach to validation should match your specific operational needs and regulatory requirements.
- If your primary focus is routine operational checks: Use a chemical indicator in every load for immediate visual confirmation, and run a test with a biological indicator at a set frequency (e.g., weekly or monthly) to verify ongoing performance.
- If your primary focus is initial qualification or post-repair validation: You must conduct a full IQ, OQ, and PQ protocol using external thermocouples and strategically placed biological indicators to formally document the machine's capability.
- If your primary focus is GxP or regulatory compliance: Meticulously document all IQ, OQ, and PQ results for every load type, establish a recurring re-qualification schedule, and have a clear protocol for investigating any BI failures.
Ultimately, robust validation is the foundation of confidence, ensuring every cycle delivers the sterility your work depends on.
Summary Table:
| Method | Purpose | Key Tools |
|---|---|---|
| Physical Monitors | Tracks time, temperature, and pressure; maps chamber performance. | Thermocouples, Data Loggers |
| Chemical Indicators | Provides immediate, visual confirmation of exposure to heat/steam. | Indicator Strips, Autoclave Tape |
| Biological Indicators (Gold Standard) | Directly measures lethality by challenging the cycle with resistant spores. | Spore strips/vials (e.g., Geobacillus stearothermophilus) |
Ensure your laboratory's sterility assurance with KINTEK solutions.
Proper autoclave validation is critical for the integrity of your research, diagnostics, and production. KINTEK specializes in providing the high-quality lab equipment and consumables—from reliable autoclaves to certified biological indicators—that your validation protocols depend on.
Let our experts help you build a robust validation and monitoring program tailored to your specific needs and compliance requirements.
Contact our team today to discuss your autoclave validation and sterility assurance needs.
Visual Guide
Related Products
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
People Also Ask
- Where should an autoclave be located in a lab? Balance Safety and Efficiency for Optimal Workflow
- What is the temperature of autoclave in microbiology lab? Achieve Sterile Conditions with 121°C
- What is an autoclave laboratory equipment? The Ultimate Guide to Steam Sterilization
- What are the settings of autoclave in microbiology? Achieve Guaranteed Sterility for Your Lab
- What is the pressure required in an autoclave? Achieve Sterile Results with 15 PSI














