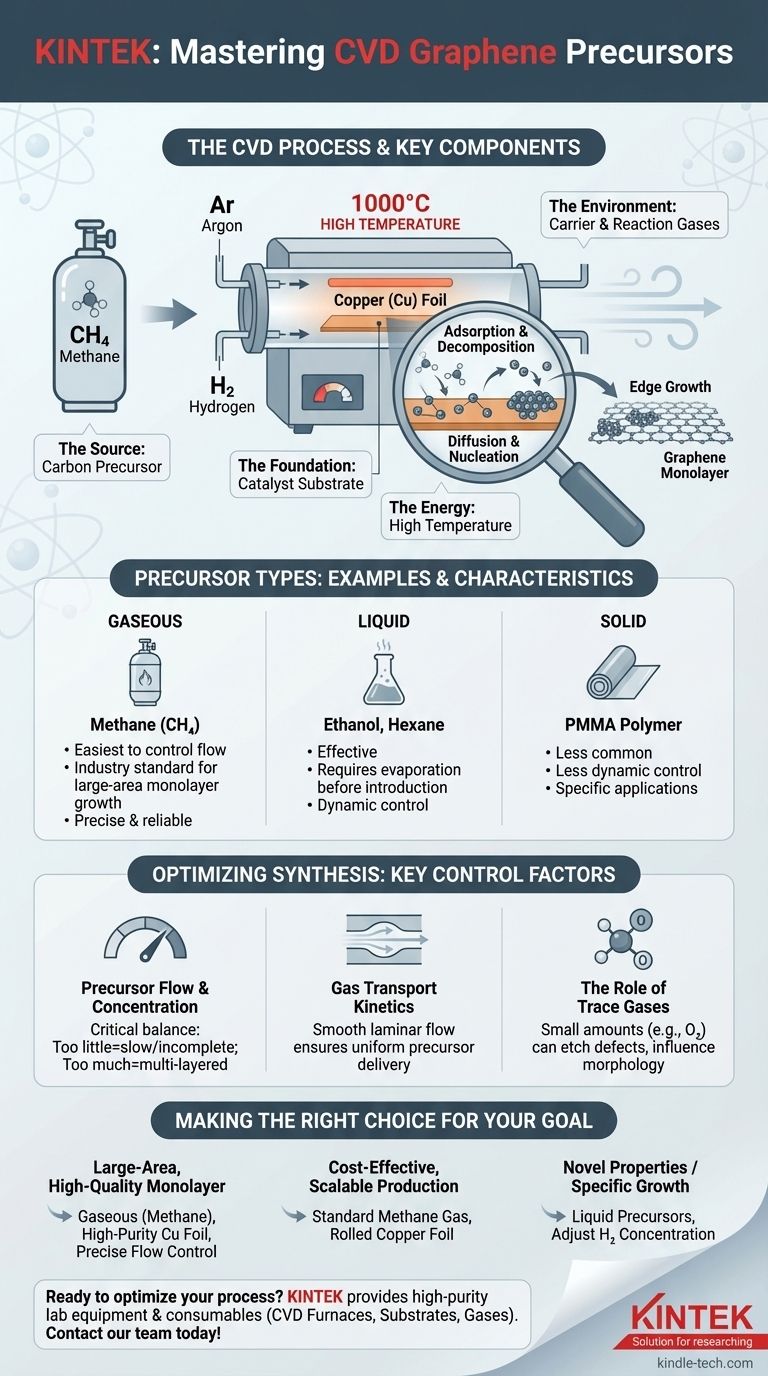
The most common precursor for producing high-quality graphene via Chemical Vapor Deposition (CVD) is a gaseous carbon source, with methane (CH4) being the industry and research standard. While liquid and solid carbon sources can also be used, methane offers the precise control and reliability needed for growing large, single-layer sheets.
The carbon precursor is the primary ingredient, but it's only one part of a precisely controlled system. Successful graphene synthesis depends equally on a catalyst substrate, specific carrier gases, and a high-temperature environment to drive the reaction.

The Core Components of Graphene CVD
To understand how a precursor becomes graphene, you must first understand the complete "recipe" and the role each component plays in the reaction.
The Carbon Precursor (The Source)
The precursor is the raw material that supplies the carbon atoms. While various hydrocarbons can work, they are typically categorized by their physical state.
Gaseous precursors, like methane, are the most widely used due to the ease of controlling their flow rate into the reaction chamber.
Liquid precursors, such as hexane or ethanol, are also effective. They are heated to evaporate and then carried into the furnace by a gas.
Solid precursors, like PMMA polymer films, can be used but offer less dynamic control over the carbon supply during the growth process.
The Catalyst Substrate (The Foundation)
Graphene growth via CVD does not happen in empty space; it requires a surface to form on. This is the role of the metal catalyst.
Copper (Cu) foil is the most common choice. Its low carbon solubility is a key advantage, naturally promoting the growth of a single layer (monolayer) of graphene. It is also inexpensive and available in large formats.
Nickel (Ni) is another common catalyst, but its higher carbon solubility can sometimes lead to the formation of multiple graphene layers, which may be undesirable.
Carrier and Reaction Gases (The Environment)
These gases create the specific atmospheric conditions needed inside the furnace. They are not passive bystanders.
Argon (Ar) is an inert gas often used to purge the system of unwanted oxygen and to maintain a stable pressure during the growth process.
Hydrogen (H2) plays a more active role. It helps keep the catalyst surface clean and can influence the shape and size of the growing graphene crystals, affecting the final quality.
High Temperature (The Energy)
The entire process takes place inside a high-temperature tubular furnace, typically heated to around 1000 °C. This extreme heat provides the energy necessary to break down the carbon precursor molecules when they come into contact with the catalyst.
How the Precursor Becomes Graphene
The transformation from a simple gas like methane into a perfect sheet of graphene is a step-by-step process at the atomic level.
Adsorption and Decomposition
First, molecules of the carbon precursor (e.g., methane) flow over the hot copper foil. The high temperature causes these molecules to break apart, or decompose, releasing individual carbon atoms onto the catalyst's surface.
Diffusion and Nucleation
These free carbon atoms are highly mobile and diffuse across the hot copper. They eventually collide with each other and begin to form stable, small clusters. This initial formation of tiny graphene crystals is called nucleation.
Edge Growth to a Monolayer
Once these initial "islands" of graphene have formed, they act as seeds. Subsequent carbon atoms arriving on the surface preferentially attach to the edges of these existing islands. This edge-growth process continues until the islands expand and merge, forming a continuous, single-atomic-layer sheet of graphene covering the entire substrate.
Understanding the Key Control Factors
Simply mixing the components is not enough. The quality of the final graphene film is extremely sensitive to the process conditions.
Precursor Flow and Concentration
The rate at which the carbon precursor is introduced is critical. Too little, and the growth is slow and may not form a complete film. Too much, and you risk forming lower-quality, multi-layered graphene.
Gas Transport Kinetics
The way gases flow through the furnace tube directly impacts the deposition process. A smooth, laminar flow is essential for ensuring the precursor is delivered uniformly across the entire catalyst surface, leading to a more consistent graphene film.
The Role of Trace Gases
Even small, sometimes unintentional, amounts of other gases like oxygen can significantly impact the final result. While often seen as a contaminant, controlled traces of oxygen can actually be used to etch away defects and influence the final morphology of the graphene grains.
Making the Right Choice for Your Goal
Understanding the precursors and their role in the larger CVD system allows you to tailor the process to your specific objective.
- If your primary focus is large-area, high-quality monolayer graphene: Use a gaseous precursor like methane with a high-purity copper foil catalyst, focusing on precise control over gas flow rates.
- If your primary focus is cost-effective, scalable production: The standard method of using methane gas on rolled copper foil remains the most economical and mature process for industrial-scale applications.
- If you are exploring novel properties or specific growth patterns: Experimenting with liquid precursors or adjusting the H2 concentration can alter the growth kinetics and final grain structure.
Mastering graphene synthesis is a matter of precisely controlling the interaction between the precursor, the catalyst, and the environment.
Summary Table:
| Precursor Type | Common Examples | Key Characteristics |
|---|---|---|
| Gaseous | Methane (CH₄) | Easiest to control, industry standard for large-area monolayer growth |
| Liquid | Ethanol, Hexane | Effective, requires evaporation before introduction |
| Solid | PMMA | Less common, offers less dynamic control during growth |
Ready to optimize your graphene synthesis process? KINTEK specializes in providing the high-purity lab equipment and consumables—from CVD furnaces to catalyst substrates and carrier gases—that are essential for reliable, high-quality graphene production. Our experts can help you select the right precursors and configure your system for success. Contact our team today to discuss your specific laboratory needs!
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- CVD Diamond Domes for Industrial and Scientific Applications
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Graphite Vacuum Continuous Graphitization Furnace
People Also Ask
- How long does it take to process a CVD diamond? A Guide to the 2-4 Week Growth Cycle
- How are thin films used? Unlock Advanced Surface Properties for Your Materials
- What are the materials for thin film technologies? Choose the Right Material for Your Application
- What are sputtering targets used for? The Essential Source for Thin-Film Manufacturing
- What is the meaning of synthesis of graphene? A Guide to Top-Down vs. Bottom-Up Methods
- Is deposition a chemical process? Understanding Chemical vs. Physical Thin-Film Methods
- What is the purpose of using electrolytic polishing on copper foils? Optimize Your CVD Graphene & hBN Growth Surface
- Can graphene be synthesized? Unlocking the Right Method for Your Application



















