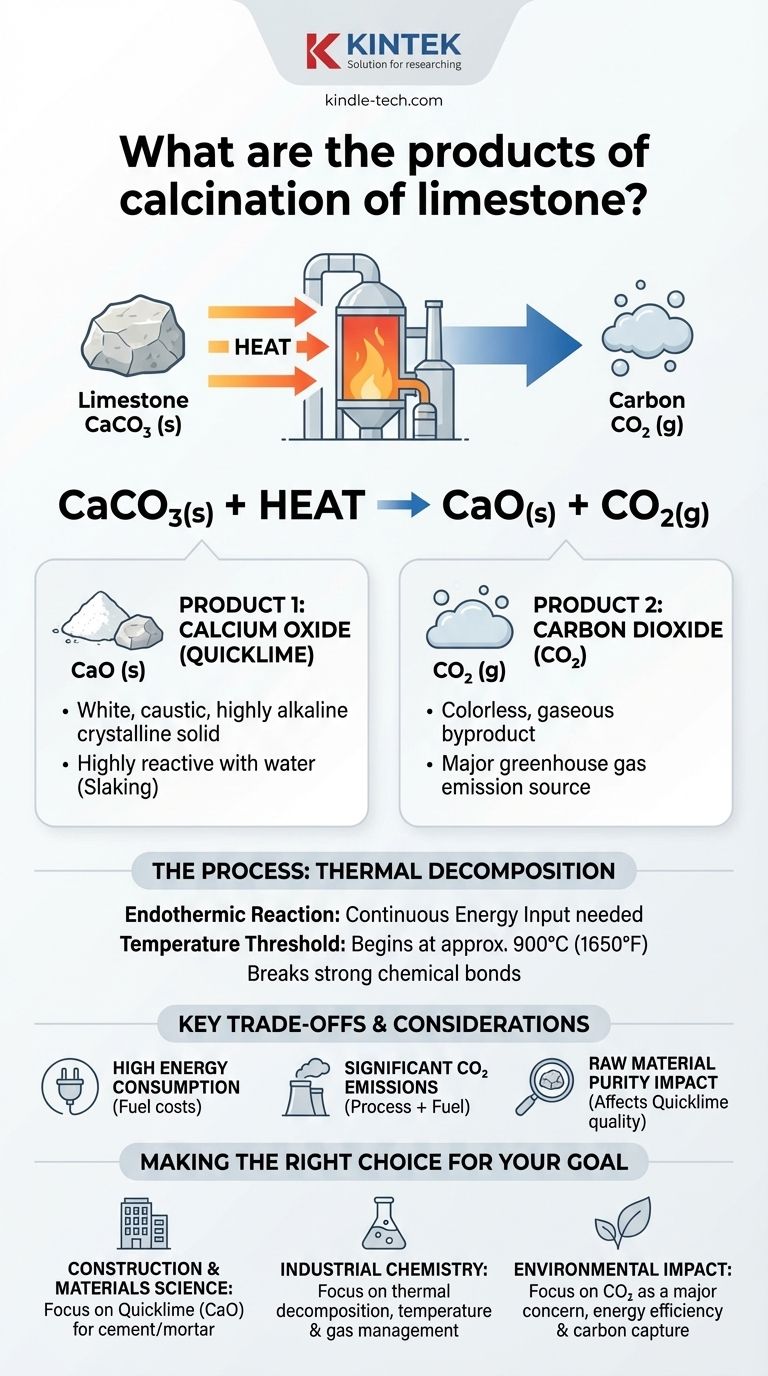
In short, the calcination of limestone produces two key products: calcium oxide (CaO) and carbon dioxide (CO₂). This process involves heating limestone, which is primarily calcium carbonate (CaCO₃), to a high temperature, causing it to chemically decompose rather than simply melt or burn.
The calcination of limestone is a foundational industrial process. It is a thermal decomposition reaction that transforms a stable, natural rock into a highly reactive chemical building block (calcium oxide) by driving off carbon dioxide gas.

The Chemistry of Calcination: A Closer Look
To fully grasp the process, it's essential to understand the underlying chemical transformation and the conditions required to make it happen.
The Core Chemical Equation
The reaction is elegantly simple and is a cornerstone of inorganic chemistry. It is an endothermic reaction, meaning it requires a continuous input of energy (heat) to proceed.
The balanced equation is: CaCO₃(s) + Heat → CaO(s) + CO₂(g)
This shows that one molecule of solid calcium carbonate breaks down into one molecule of solid calcium oxide and one molecule of gaseous carbon dioxide.
The Role of Thermal Decomposition
Calcination is not a reaction with heat; it's a decomposition caused by heat. The thermal energy is used to break the strong chemical bonds within the calcium carbonate structure.
Critical Temperature Threshold
The decomposition of calcium carbonate begins at approximately 900°C (1650°F) under normal atmospheric pressure. In industrial kilns, temperatures are often kept higher to ensure the reaction proceeds efficiently and completely.
Understanding the Products and Their Properties
The two products of this reaction have vastly different characteristics and uses, which is why the process is so fundamental to industry.
Product 1: Calcium Oxide (Quicklime)
Calcium oxide, commonly known as quicklime or burnt lime, is the primary desired product. It is a white, caustic, and highly alkaline crystalline solid.
Its most important property is its high reactivity, especially with water. This exothermic reaction, called slaking, produces calcium hydroxide, or hydrated lime.
Product 2: Carbon Dioxide (CO₂)
Carbon dioxide is the gaseous byproduct that is driven off and removed from the kiln during the process.
While sometimes captured for other industrial uses, it is more often considered a waste product and a significant source of greenhouse gas emissions.
Understanding the Trade-offs and Key Considerations
While the process is straightforward chemically, its industrial application involves significant challenges and impacts that must be managed.
High Energy Consumption
Achieving and maintaining temperatures above 900°C is an extremely energy-intensive process. Fuel costs represent a major portion of the operational expense of producing quicklime.
Significant CO₂ Emissions
The calcination of limestone is a major global source of carbon dioxide emissions. The CO₂ comes from two sources: the chemical decomposition of the limestone itself and the fossil fuels typically burned to heat the kilns.
Impact of Raw Material Purity
The quality of the final quicklime is directly dependent on the purity of the initial limestone. Impurities such as silica, clay, or magnesium carbonate can affect the reactivity and properties of the final product, which is critical for applications like steel and cement manufacturing.
Making the Right Choice for Your Goal
Understanding this process allows you to appreciate its role in different fields. The importance of the products depends entirely on your specific application.
- If your primary focus is construction and materials science: The key product is calcium oxide (quicklime), as it is the essential precursor to cement and mortar.
- If your primary focus is industrial chemistry: View this as a classic thermal decomposition reaction where managing temperature, energy inputs, and gas removal are the critical process variables.
- If your primary focus is environmental impact: Recognize that the byproduct, carbon dioxide, is a major concern, making energy efficiency and carbon capture technologies vital for the industry's future.
Ultimately, understanding the products of limestone calcination is the first step toward mastering its application across countless essential industries.
Summary Table:
| Product | Chemical Formula | Common Name | Key Properties |
|---|---|---|---|
| Calcium Oxide | CaO | Quicklime | White, caustic, highly alkaline, reactive with water |
| Carbon Dioxide | CO₂ | - | Colorless gas, greenhouse gas, industrial byproduct |
Optimize your calcination process with KINTEK's advanced laboratory equipment. Whether you're researching thermal decomposition reactions or scaling up industrial production, our high-temperature furnaces and kilns ensure precise temperature control and energy efficiency. We provide the reliable tools you need to produce high-purity quicklime while managing energy consumption and emissions. Contact us today (#ContactForm) to discuss how our solutions can enhance your materials science and industrial chemistry workflows.
Visual Guide
Related Products
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
People Also Ask
- What is the activation temperature of activated carbon? A Guide to Method, Temperature, and Pore Structure
- What is the purpose of the rotary kiln? A Guide to Continuous High-Temperature Processing
- What is the thermal regeneration process of activated carbon? Restore Performance and Cut Costs
- What is pyrolysis mechanism of biomass materials? A Guide to Converting Biomass into Biochar, Bio-oil, and Syngas
- What is microwave pyrolysis? Unlock Faster, More Efficient Waste-to-Value Conversion
- What is biochar obtained from the pyrolysis? A Key Product of Biomass Conversion
- What is the temperature of a rotary kiln? It's a Controlled Thermal Journey, Not a Single Number
- What is the process of rotary kiln? Achieve Precise Industrial Material Transformation



















