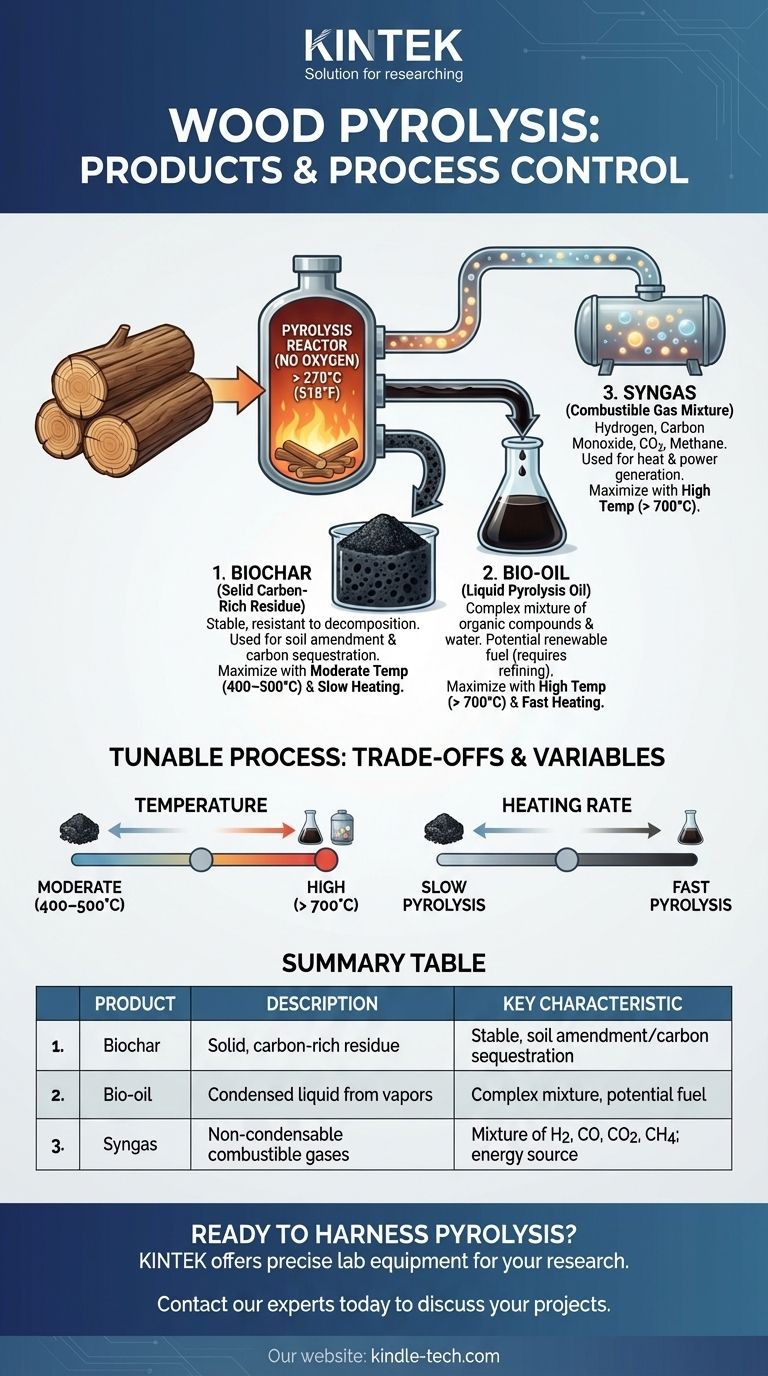
In short, the pyrolysis of wood yields three primary products. In the absence of oxygen, heating wood to high temperatures breaks it down into a solid carbon-rich residue called biochar, a liquid known as bio-oil (or pyrolysis oil), and a mixture of combustible gases called syngas.
The most critical insight is that pyrolysis is not a single, fixed reaction. It is a tunable process where adjusting factors like temperature and heating rate allows you to deliberately favor the production of either the solid, the liquid, or the gaseous products.

The Three Core Products of Wood Pyrolysis
When wood is heated above 270°C (518°F) without oxygen, its complex organic structures begin to decompose. This process, known as carbonization, separates the material into distinct physical states.
The Solid: Biochar
Biochar is the stable, carbon-rich solid that remains after the volatile components have been driven off. It is the material most people recognize as charcoal.
This solid residue is primarily elemental carbon, which is why it is so resistant to further decomposition. Its properties can be further refined by heating it to temperatures above 600°C.
The Liquid: Bio-oil
As the wood decomposes, many of its organic compounds vaporize. When these hot vapors are cooled and condensed, they form a dark, dense liquid known as bio-oil.
Bio-oil is a complex mixture of water and hundreds of different organic compounds. It is considered a potential renewable fuel, though it often requires upgrading before use.
The Gas: Syngas
The non-condensable components of the pyrolysis vapor form the gas product, or syngas.
This is a mixture of combustible gases, primarily including hydrogen, carbon monoxide, carbon dioxide, and methane. This gas can be captured and burned to generate heat or electricity, often to help power the pyrolysis process itself.
How Process Conditions Dictate the Outcome
You cannot maximize the yield of all three products simultaneously. The final distribution of biochar, bio-oil, and syngas is a direct result of the specific process conditions you choose.
The Decisive Role of Temperature
Temperature is the most powerful lever you can pull to influence the outcome. There is a clear and predictable relationship between heat and the final product yields.
A moderate temperature range of 400–500°C (752–932°F) limits the breakdown of the solid carbon structure, thus maximizing the production of biochar.
Conversely, high temperatures above 700°C (1292°F) aggressively break down the wood into smaller volatile molecules, favoring the production of liquid and gaseous fuels.
The Influence of Heating Rate
The speed at which the wood is heated also plays a critical role.
A slow pyrolysis process, where heat is applied gradually over a longer period, allows more time for carbon structures to form and stabilize. This method is ideal for producing high-quality biochar while minimizing the yield of volatile gases.
A fast pyrolysis process, in contrast, rapidly vaporizes the biomass, maximizing the production of bio-oil.
The Absolute Requirement: No Oxygen
It is crucial to remember that pyrolysis is defined by the absence of oxygen. If oxygen is present, the wood will simply combust (burn), producing heat, smoke, and a small amount of mineral ash, not biochar.
Understanding the Trade-offs
Choosing a pyrolysis strategy involves accepting a fundamental set of trade-offs. Optimizing for one product comes at the expense of another.
The Yield Dilemma
There is a direct competition between the products. A process designed to maximize biochar yield (slow heating, moderate temperature) will inherently produce less bio-oil and syngas.
Conversely, a high-yield liquid fuel process (fast heating, high temperature) will leave behind a much smaller amount of solid biochar.
Product Quality and Complexity
The products are not always immediately usable. Bio-oil, for instance, is a tar-like, acidic, and often unstable mixture that typically requires significant and costly refining before it can be used as a direct replacement for conventional fuels.
Making the Right Choice for Your Goal
The "best" pyrolysis method depends entirely on what product you value most. You must match the process conditions to your desired outcome.
- If your primary focus is producing a solid soil amendment or carbon-sequestering material: Use slow pyrolysis at moderate temperatures (400–500°C) to maximize the yield and quality of biochar.
- If your primary focus is creating liquid or gaseous fuels: Use fast pyrolysis at high temperatures (above 700°C) to rapidly break down the wood into its volatile components.
Ultimately, understanding these variables transforms pyrolysis from a simple decomposition into a precise tool for creating valuable materials.
Summary Table:
| Product | Description | Key Characteristic |
|---|---|---|
| Biochar | Solid, carbon-rich residue | Stable, used for soil amendment/carbon sequestration |
| Bio-oil | Condensed liquid from vapors | Complex mixture, potential renewable fuel source |
| Syngas | Non-condensable combustible gases | Mixture of H2, CO, CO2, CH4; used for energy |
Ready to harness the power of pyrolysis for your research or production needs?
At KINTEK, we specialize in high-quality lab equipment, including pyrolysis reactors, designed to give you precise control over temperature and process conditions. Whether your goal is to maximize biochar production or optimize for bio-oil and syngas, our solutions are built to deliver reliable, tunable results for your laboratory.
Contact our experts today to discuss how we can support your specific pyrolysis projects and help you create valuable materials from biomass.
Visual Guide
Related Products
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
People Also Ask
- What is the difference between calcining and roasting? A Guide to High-Temperature Processing
- What are the industrial applications of pyrolysis? Transform Waste into Energy and Valuable Products
- What is the principle of rotary kiln? Mastering Continuous Thermal Processing
- What equipment is used in pyrolysis? Choosing the Right Reactor for Your Feedstock and Products
- What are the zones in rotary kiln in cement production? Master the Core Process for High-Quality Clinker



















