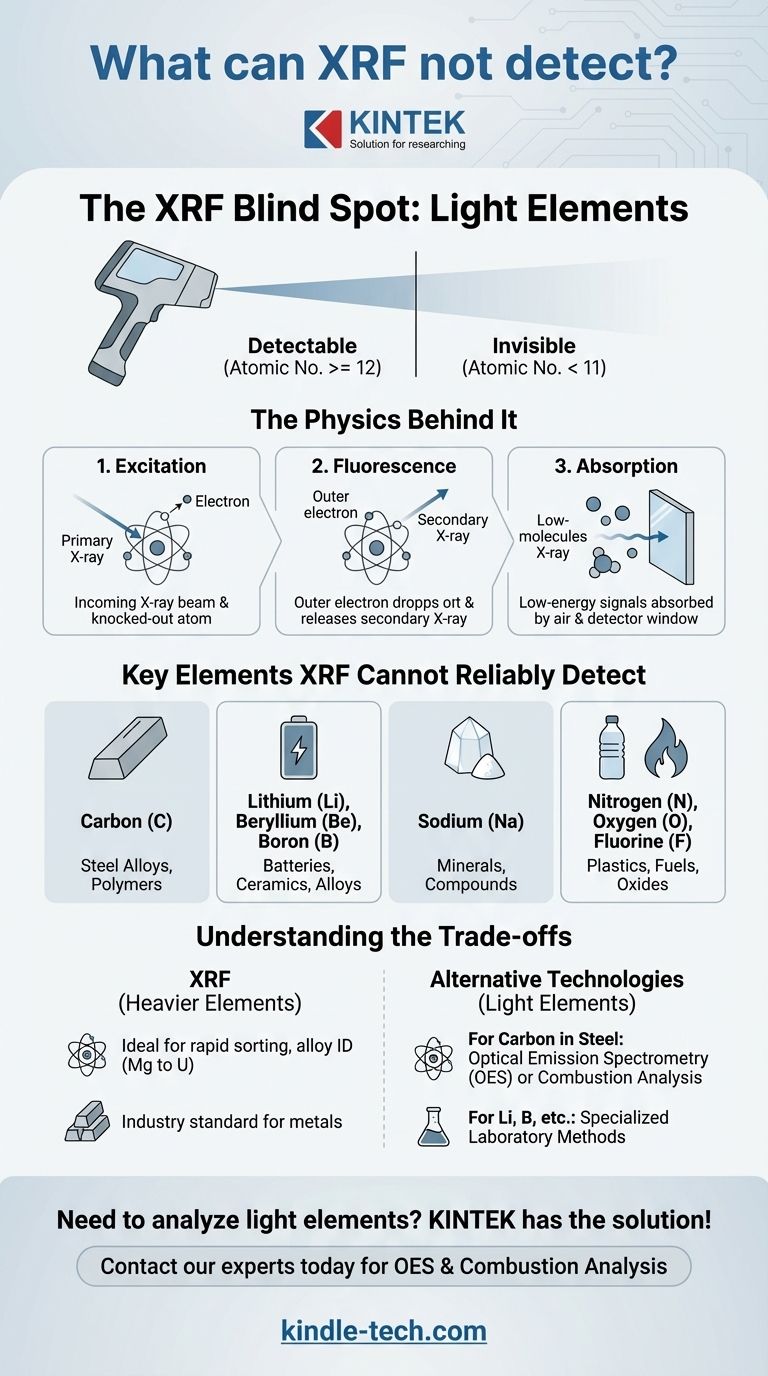
At its core, X-Ray Fluorescence (XRF) technology cannot detect very light elements. The standard detection limit for most handheld analyzers begins at magnesium (Mg), element number 12 on the periodic table. This means any element with an atomic number of 11 or lower is effectively invisible to standard XRF analysis.
The inability of XRF to detect light elements is not a flaw in the equipment, but a fundamental constraint of physics. The very weak, low-energy signals produced by these elements are absorbed by the air before they can even reach the analyzer's detector.

Why XRF Has an Elemental Blind Spot
To understand the limitations of XRF, you first have to understand how it works. The technology is based on the unique energy signature each element releases after being excited by an X-ray source.
The Physics of Fluorescence
An XRF analyzer directs a primary X-ray beam at a sample. This beam strikes atoms within the material, knocking an electron out of an inner orbital shell.
To regain stability, an electron from a higher-energy outer shell immediately drops down to fill the vacancy. This transition releases a specific amount of energy in the form of a secondary X-ray, which is called fluorescence.
Because the energy spacing between electron shells is unique for every element, the energy of this fluorescent X-ray acts as a distinct "fingerprint." The analyzer's detector measures these fingerprints to identify which elements are present and in what quantity.
The Low-Energy Problem
The energy of a fluorescent X-ray is directly proportional to the element's atomic number. Heavy elements like uranium produce high-energy X-rays that travel easily and are simple to detect.
Conversely, light elements produce very low-energy (long-wavelength) fluorescent X-rays. Elements like carbon, sodium, and lithium emit such weak signals that they are difficult or impossible for the detector to register reliably.
Detection and Absorption Challenges
The primary obstacle for these low-energy X-rays is the air itself. The weak signal is easily absorbed by air molecules in the short distance between the sample and the analyzer's detector.
Furthermore, even the protective window on the detector (typically made of beryllium) can absorb the weakest of these signals. This combination of factors creates a practical detection floor at magnesium for most field-portable units.
Key Elements XRF Cannot Reliably Detect
While the rule is "elements lighter than magnesium," it's important to recognize the specific, industrially-relevant materials that fall into this category.
Carbon (C)
This is arguably the most significant limitation of XRF in metallurgy. XRF cannot determine the carbon content in steel, which is the primary element that defines the grade and properties of carbon steel, stainless steel, and other alloys.
Lithium (Li), Beryllium (Be), and Boron (B)
These are extremely light elements critical to modern industries. Lithium is essential for batteries, while beryllium and boron are used in specialized alloys and high-tech applications. XRF cannot be used to identify or quantify them.
Sodium (Na)
As element 11, sodium is the element immediately preceding magnesium. It is a common element in many minerals and materials that XRF will not be able to see.
Nitrogen (N), Oxygen (O), and Fluorine (F)
These non-metals are foundational to countless chemical compounds, polymers, and minerals. XRF is not a suitable tool for analyzing their presence.
Understanding the Trade-offs
Recognizing what XRF cannot do is just as important as knowing what it can. This allows you to select the right analytical tool for the job and avoid costly errors.
A Tool for Heavier Elements
The limitation regarding light elements does not diminish XRF's power for its intended purpose. It remains the industry standard for the rapid sorting, identification, and quality control of thousands of metal alloys based on their content of chromium, nickel, copper, tungsten, titanium, and other elements from magnesium to uranium.
When to Use a Different Technology
If your application requires measuring carbon or other light elements, you must use a different technology. For carbon in steel, the definitive methods are Optical Emission Spectrometry (OES) or combustion analysis.
It's a Limitation, Not an Absence
It is critical to remember that just because an XRF analyzer does not report an element like carbon, it does not mean it isn't there. It simply means the technology is physically incapable of detecting it.
Making the Right Choice for Your Goal
Selecting the correct analytical instrument depends entirely on the question you need to answer.
- If your primary focus is rapidly sorting common scrap metal or identifying alloys like stainless steel or nickel superalloys: XRF is the ideal, non-destructive tool for the job.
- If your primary focus is determining the exact carbon grade of a steel component for quality assurance: You must use a technology like mobile OES, as XRF cannot provide this information.
- If your primary focus is analyzing for lithium, boron, or other elements lighter than magnesium: You will need to explore alternative laboratory methods suited for these specific light elements.
Ultimately, understanding the inherent physical boundaries of XRF is the first step toward using it effectively and knowing when to rely on a different tool to get the right answer.
Summary Table:
| Elements XRF Cannot Detect | Atomic Number | Common Applications |
|---|---|---|
| Carbon (C) | 6 | Steel alloys, polymers |
| Lithium (Li) | 3 | Batteries, ceramics |
| Sodium (Na) | 11 | Minerals, compounds |
| Nitrogen (N), Oxygen (O) | 7, 8 | Plastics, fuels, oxides |
| Boron (B), Beryllium (Be) | 5, 4 | Alloys, nuclear materials |
Need to analyze light elements like carbon in steel or lithium in batteries? XRF has its limits, but KINTEK has the solution. As your trusted lab equipment partner, we provide a full range of analytical technologies—including Optical Emission Spectrometry (OES) and combustion analyzers—to accurately detect elements XRF cannot. Ensure your material analysis is complete and reliable. Contact our experts today to find the perfect tool for your specific application and get precise results for all your elements, light or heavy.
Visual Guide
Related Products
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Single Punch Tablet Press Machine and Mass Production Rotary Tablet Punching Machine for TDP
- Single Punch Manual Tablet Press Machine TDP Tablet Punching Machine
- Small Injection Molding Machine for Lab Use
- Mini Planetary Ball Mill Machine for Laboratory Milling
People Also Ask
- Why use a vibratory sieve shaker for PET powder? Achieve Precise Particle Size Control for Chemical Research
- Why is a standardized sieving system necessary for elephant grass research? Ensure Reliable Sample Consistency
- What function does a sieving system perform during HPS powder pretreatment? Ensure Uniform Particle Size Distribution
- Why is powder classification using standard sieves essential for SHS reactions? Unlock Superior Nitriding Results
- What are the disadvantages of sieve machine? Key Limitations in Particle Size Analysis



















