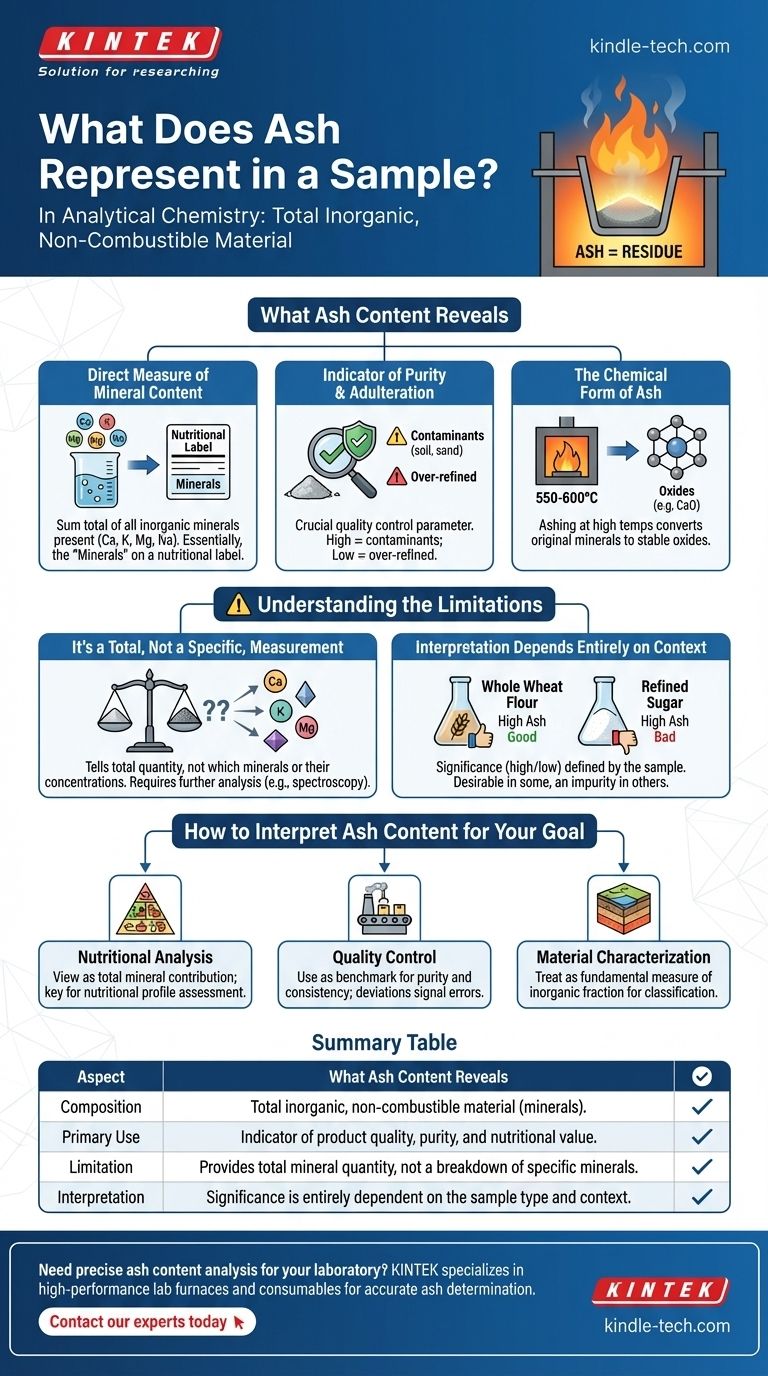
In analytical chemistry, ash represents the total amount of inorganic, non-combustible material present in a sample. It is the residue that remains after the organic matter has been completely burned away at high temperatures. This residue consists primarily of the oxides of essential minerals and trace elements that were part of the original sample.
Measuring ash is not simply about what is left after burning; it is a fundamental indicator of a product's quality, purity, and nutritional value. It provides a direct measure of the total mineral content within a sample.

What Ash Content Reveals About a Sample
Understanding the ash value of a substance provides critical insight into its fundamental composition. It serves as a key metric in fields ranging from food science to geology.
A Direct Measure of Mineral Content
Ash is the sum total of all the minerals present. These are the inorganic elements that organisms cannot create, such as calcium, potassium, magnesium, and sodium.
When you see "Minerals" on a nutritional label, you are essentially looking at a representation of the ash content.
An Indicator of Purity and Adulteration
For many processed products, ash content is a crucial quality control parameter. An unusually high ash value can indicate the presence of contaminants like soil, sand, or other inorganic adulterants.
Conversely, an ash value that is too low might suggest that a product has been overly refined, stripping it of its natural mineral content.
The Chemical Form of Ash
The process of determining ash content, known as ashing, involves combustion at very high temperatures (typically 550-600°C).
At these temperatures, the original minerals are converted into their more chemically stable oxide forms. For example, calcium becomes calcium oxide. Therefore, the ash itself is a collection of these oxides.
Understanding the Limitations
While ash analysis is a powerful tool, it is essential to recognize its limitations to interpret the data correctly. It provides a total quantity, not a detailed breakdown.
It's a Total, Not a Specific, Measurement
Ash content tells you the total amount of inorganic material, but it does not identify which specific minerals are present or their individual concentrations.
A sample could have a high ash value from beneficial minerals like calcium and potassium, or from less desirable elements. Further analysis, such as spectroscopy, would be required to determine the specific mineral profile.
Interpretation Depends Entirely on Context
A "high" or "low" ash content is not inherently good or bad. Its significance is defined by the sample being tested.
For example, high ash in whole wheat flour is desirable, as it indicates a higher presence of the mineral-rich bran. In contrast, any significant ash content in refined white sugar would be considered an impurity.
How to Interpret Ash Content for Your Goal
The significance of an ash value is entirely dependent on your objective. Use this metric as a specific lens to evaluate your sample.
- If your primary focus is nutritional analysis: View ash content as the total mineral contribution of a food or feed, a key factor in assessing its overall nutritional profile.
- If your primary focus is quality control: Use ash content as a benchmark for purity and consistency, where deviations from a standard can signal contamination or processing errors.
- If your primary focus is material characterization: Treat ash as the fundamental measure of the inorganic fraction of a substance, essential for identifying and classifying materials like coal or soil.
Ultimately, understanding ash content moves beyond a simple definition to become a powerful tool for evaluating the true composition and quality of a sample.
Summary Table:
| Aspect | What Ash Content Reveals |
|---|---|
| Composition | Total amount of inorganic, non-combustible material (minerals). |
| Primary Use | Indicator of product quality, purity, and nutritional value. |
| Limitation | Provides total mineral quantity, not a breakdown of specific minerals. |
| Interpretation | Significance (high/low) is entirely dependent on the sample type and context. |
Need precise ash content analysis for your laboratory?
Accurate ashing is fundamental for reliable quality control, nutritional labeling, and material characterization. KINTEK specializes in high-performance lab furnaces and consumables designed for precise and consistent ash determination, ensuring your results are trustworthy.
Let us help you enhance your analytical capabilities. Contact our experts today to find the perfect equipment for your specific application and sample type.
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the alternative to a laboratory oven? Find the Right Heating Tool for Your Lab
- What precautions should you take while using a muffle furnace? Ensure Safe High-Temperature Processing in Your Lab
- How do high-temperature laboratory furnaces assist in enhancing C/C composites? Achieve 7.5x Better Corrosion Resistance
- What is the use of a heat treatment oven? Transform Material Properties for Superior Performance
- What is the importance of ash determination in foods? A Key to Quality, Nutrition & Purity
- What function does a box muffle furnace serve in LiNbO3 coatings on NCA? Enhance Cathode Interface Stability
- Why is a high-temperature muffle furnace necessary for POM desulfurization catalysts? Master Catalyst Activation
- What is the application of sintering furnace? Transform Powder into High-Performance Components



















