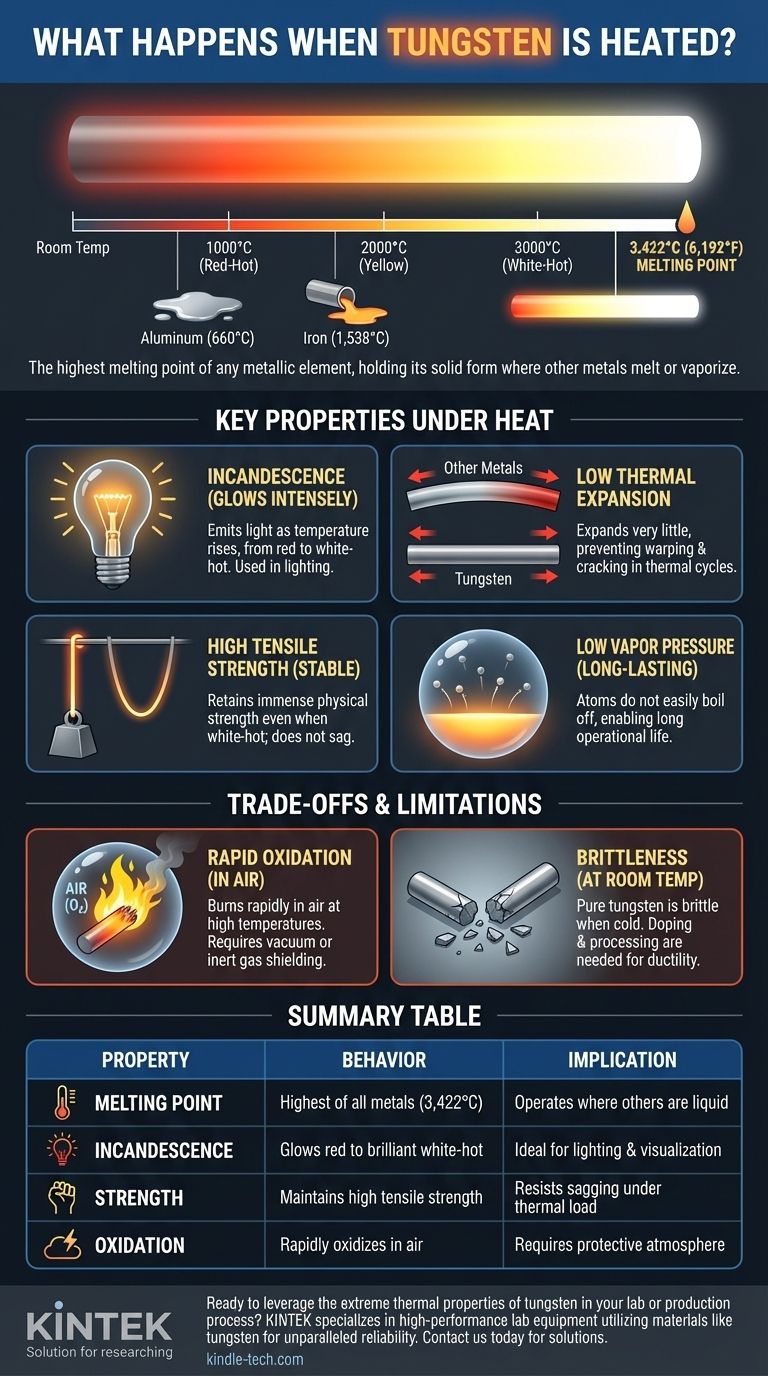
In short, when tungsten is heated, it glows intensely and maintains its solid form at temperatures that would melt or vaporize nearly any other metal. It has the highest melting point of any metallic element, 3,422 °C (6,192 °F), and only begins to boil at an astonishing 5,930 °C (10,706 °F). This extraordinary thermal stability is the foundation of its most critical industrial uses.
The true value of tungsten is not just its high melting point, but its unique ability to remain physically strong and stable at extreme temperatures. Where other metals weaken and deform, tungsten holds its shape, making it indispensable for high-performance applications.
The Defining Characteristic: An Exceptionally High Melting Point
How High Is It?
Tungsten's melting point of 3,422 °C is in a class of its own.
To put this in perspective, iron melts at 1,538 °C, and aluminum melts at a mere 660 °C. Tungsten can operate effectively in environments where steel would be a flowing liquid.
What This Means in Practice
This property allows tungsten to be used as a primary material for components that must function in extreme thermal environments, such as rocket engine nozzles, heating elements in high-temperature furnaces, and radiation shielding.
Key Properties Under Heat
Heating tungsten reveals several other crucial behaviors beyond simply resisting melting. These properties work in concert to make it so uniquely useful.
Incandescence: The Ability to Glow
Long before it melts, tungsten will glow brightly when heated, a phenomenon known as incandescence.
As the temperature rises, the color of the light it emits shifts from red to orange, then to yellow, and finally to a brilliant white-hot. This is the exact principle that allows an incandescent light bulb filament to produce visible light.
Low Thermal Expansion
Tungsten has a very low coefficient of thermal expansion. This means it expands and contracts very little when its temperature changes dramatically.
This stability is critical for precision components. It prevents the material from warping, cracking, or breaking under the mechanical stress of repeated heating and cooling cycles.
High Tensile Strength at High Temperatures
Perhaps its most important non-obvious trait is that tungsten retains incredible physical strength even when white-hot.
Most metals become extremely soft and malleable (a state often called "red-short") long before they melt. Tungsten, however, remains rigid and strong, allowing it to maintain a precise shape, like a thin filament or an electrode tip, without sagging or deforming.
Low Vapor Pressure
Even at temperatures approaching its melting point, tungsten atoms do not easily "boil" off the surface. This property, known as low vapor pressure, is why a lightbulb filament can last for over a thousand hours of operation without simply evaporating away.
Understanding the Trade-offs and Limitations
Tungsten's remarkable heat resistance is not without its challenges. Understanding these limitations is key to using it effectively.
Rapid Oxidation in Air
This is tungsten's primary vulnerability. While it withstands heat, it does not withstand oxygen at high temperatures.
When heated in the presence of air, tungsten will rapidly oxidize and burn, forming a yellow tungsten trioxide powder. This is why high-temperature applications like lightbulbs or TIG welding require the tungsten to be shielded in a vacuum or an inert gas atmosphere (like argon).
Brittleness at Room Temperature
In its pure state, tungsten is very brittle at room temperature. A pure tungsten rod can shatter like a piece of ceramic if dropped.
To create useful products like ductile wires for filaments, it must be doped with other elements and undergo a complex manufacturing process of sintering, swaging, and drawing to achieve the desired mechanical properties.
Applying This to Your Goal
Your choice to use tungsten should be based on a clear understanding of its unique profile.
- If your primary focus is creating light from heat: Tungsten's incandescence and high melting point are ideal, but you must use it in a vacuum or inert gas to prevent oxidation.
- If your primary focus is high-temperature structural integrity: Its high tensile strength and low thermal expansion make it perfect for furnace parts or aerospace components that must not deform under extreme heat.
- If your primary focus is a durable electrical contact point: Its resistance to melting and electrical erosion makes it a superior choice for welding electrodes or high-energy spark gaps, again, provided it's shielded from oxygen.
By understanding both its extreme strengths and its specific weaknesses, you can effectively harness tungsten for the world's most demanding high-temperature applications.
Summary Table:
| Property Under Heat | Behavior of Tungsten | Key Implication |
|---|---|---|
| Melting Point | Highest of all metals (3,422 °C / 6,192 °F) | Can operate where other metals are liquid |
| Incandescence | Glows from red to brilliant white-hot | Ideal for lighting and high-temperature visualization |
| Strength | Maintains high tensile strength even when white-hot | Resists sagging and deformation under extreme thermal loads |
| Oxidation | Rapidly oxidizes and burns in air at high temperatures | Requires a vacuum or inert gas atmosphere for protection |
Ready to leverage the extreme thermal properties of tungsten in your lab or production process?
KINTEK specializes in high-performance lab equipment and consumables that utilize materials like tungsten for unparalleled reliability. Whether you need durable furnace components, specialized electrodes, or expert advice on material selection for high-temperature environments, our team is here to ensure your success.
Contact KINTEK today to discuss how our tungsten-based solutions can enhance your application's performance and durability.
Visual Guide
Related Products
- Thermally Evaporated Tungsten Wire for High Temperature Applications
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Boron Nitride (BN) Ceramic Rod for High Temperature Applications
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Automatic High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
People Also Ask
- Which is better quartz or ceramic heating element? Choose the Right Heat for Your Application
- How can you tell if a heating element is bad? Diagnose with a Multimeter in 4 Steps
- What is silicon carbide rod heated to high temperature used as? A Premier Heating Element for Extreme Environments
- What is the purpose of using a Pt-Rh thermocouple in magnesium experiments? Ensure Precise Vapor Collection
- What is the function of glass-ceramic heaters in high-temperature evaporation? Ensure Thermal Precision & Stability
- Can tungsten withstand high temperature? Unlocking its full potential in extreme heat environments
- How does precision temperature-controlled heating equipment facilitate Cu3N to MCL conversion? Optimize MCL Synthesis
- How does the combination of thermocouples and temperature control systems affect the study of reduction kinetics?

















