The short answer is there is no single instrument to measure vacuum. The correct tool, known as a vacuum gauge, depends entirely on the level of vacuum you need to measure, as the physics of measurement must change as the number of gas molecules decreases.
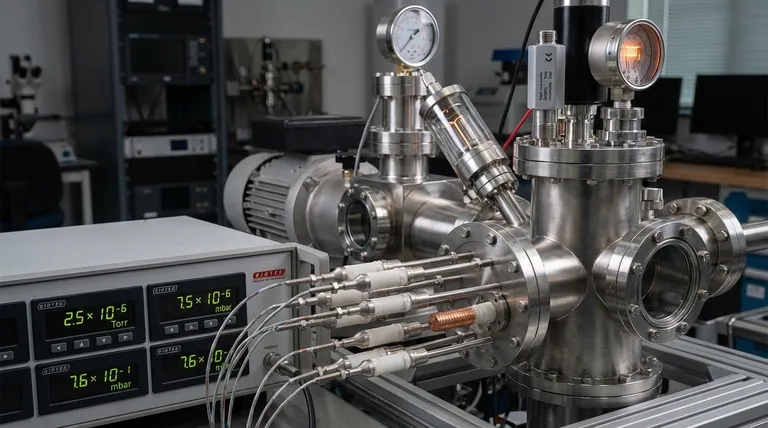
The central challenge in vacuum measurement is that a single gauge cannot span the entire pressure range from atmospheric down to near-perfect vacuum. The right instrument is always a direct match for the specific vacuum level, determined by which physical property of the residual gas is most practical to measure.
Why One Gauge Isn't Enough: Understanding Vacuum Ranges
To select the right gauge, you must first understand that "vacuum" isn't a single state but a vast spectrum of pressures, each requiring a different measurement approach.
What is a Vacuum?
A vacuum is any space where the gas pressure is lower than the surrounding atmospheric pressure. It's a measure of the absence of matter.
The Key Vacuum Ranges
Engineers and scientists typically divide the vacuum spectrum into several ranges. As pressure drops, the number of gas molecules per cubic centimeter plummets, forcing a change in measurement strategy.
- Low Vacuum (Rough Vacuum): ~1 to 760 Torr (atmospheric pressure).
- Medium Vacuum: ~10⁻³ to 1 Torr.
- High Vacuum (HV): ~10⁻⁹ to 10⁻³ Torr.
- Ultra-High Vacuum (UHV): Below 10⁻⁹ Torr.
How Gauges Work: Direct vs. Indirect Measurement
The fundamental difference between gauges lies in whether they measure the pressure directly or infer it from another property. This is the most important concept for choosing the right tool.
Direct Gauges: Measuring Physical Force
In low vacuum ranges, there are enough gas molecules to exert a measurable physical force. Direct gauges measure this force.
These gauges are gas-type independent, meaning their readings are accurate regardless of the gas being measured (e.g., air, argon, helium). A common example is the capacitance manometer.
Indirect Gauges: Inferring Pressure from Gas Properties
In high and ultra-high vacuum, there are too few molecules to exert a detectable force. Instead, indirect gauges measure a gas property that changes predictably with pressure, such as thermal conductivity or ionization probability.
These gauges are gas-type dependent and are typically calibrated for nitrogen or air. Using them with other gases requires applying a correction factor to get an accurate reading.
Common Types of Vacuum Gauges Explained
Each type of gauge is designed to operate within a specific pressure range where its underlying physical principle is most effective.
For Low Vacuum: Pirani & Thermocouple Gauges
These are thermal conductivity gauges. They work by heating a wire filament and measuring how much heat it loses to the surrounding gas.
More gas molecules (higher pressure) pull away more heat, cooling the wire. Fewer molecules (lower pressure) result in less heat loss. This change is correlated to a pressure reading. They are excellent for monitoring the initial pump-down of a system.
For High Vacuum: Hot-Cathode Ionization Gauges
Once the pressure is too low for thermal effects to be useful, ionization gauges take over. A hot filament emits electrons, which fly through the vacuum and collide with the few remaining gas molecules, creating positive ions.
The gauge measures the resulting electrical current from these ions. A higher ion current means more gas molecules are present, indicating a higher pressure. The Bayard-Alpert gauge is a very common type.
For High & Ultra-High Vacuum: Cold-Cathode Gauges
Also known as Penning gauges, these operate similarly to hot-cathode gauges by measuring ion current. However, they use a high-voltage discharge within a magnetic field to create ions instead of a heated filament.
This makes them more rugged and less prone to burnout, but they are generally less accurate than their hot-cathode counterparts.
Understanding the Practical Trade-offs
Selecting a gauge isn't just about the pressure range; it involves understanding critical limitations that can affect your measurements and the health of your system.
Gas Composition Dependency
This is the most common pitfall with indirect gauges (Pirani, Ionization). If your system is filled with argon but your gauge is calibrated for air, the reading will be incorrect. Always know what your gauge is calibrated for and apply the correct conversion factor if necessary.
Contamination and Burnout
Hot-cathode ionization gauges are sensitive. Operating them at too high a pressure (above 10⁻³ Torr) will rapidly burn out the filament. They can also be contaminated by process gases, which alters their accuracy.
The Crossover Problem
Because no single gauge covers the full spectrum, most vacuum systems require at least two types: one for the initial "roughing" stage (like a Pirani) and another for the high-vacuum stage (like an ionization gauge). Managing the transition between these gauges is a key part of vacuum system operation.
Selecting the Right Gauge for Your Application
Your choice should be dictated by your ultimate goal and the specific pressure range you need to control or monitor.
- If your primary focus is initial pump-down (rough vacuum): A Pirani or thermocouple gauge is the robust, cost-effective choice.
- If your primary focus is monitoring a high-vacuum process (e.g., coating or analysis): A hot-cathode (Bayard-Alpert) ionization gauge provides the necessary accuracy in the high-vacuum range.
- If you need a rugged gauge for an industrial high-vacuum process: A cold-cathode (Penning) gauge offers reliability and a long lifespan.
- If you require gas-independent accuracy for process control in the low-to-medium range: A capacitance manometer is the definitive standard.
Ultimately, understanding how a gauge works is the key to trusting its measurement and achieving your goal.
Summary Table:
| Vacuum Range | Pressure (Torr) | Primary Gauge Type | Key Principle |
|---|---|---|---|
| Low (Rough) Vacuum | 1 to 760 | Pirani / Thermocouple | Thermal Conductivity |
| Medium Vacuum | 10⁻³ to 1 | Capacitance Manometer | Direct Force Measurement |
| High Vacuum (HV) | 10⁻⁹ to 10⁻³ | Hot-Cathode Ionization | Ionization Current |
| Ultra-High Vacuum (UHV) | < 10⁻⁹ | Cold-Cathode (Penning) | Ionization in Magnetic Field |
Need Expert Help Selecting the Right Vacuum Gauge?
Choosing the correct vacuum gauge is critical for the accuracy and reliability of your laboratory processes. The wrong choice can lead to measurement errors, equipment damage, and process failures.
KINTEK is your trusted partner for all laboratory equipment needs. We specialize in providing the precise vacuum gauges and complete vacuum solutions your lab requires. Our experts will help you:
- Select the ideal gauge for your specific pressure range and application (e.g., coating, analysis, R&D).
- Avoid common pitfalls like gas composition errors and gauge burnout.
- Ensure optimal performance and longevity for your vacuum systems.
Don't leave your vacuum measurements to chance. Contact KINTEK today for a consultation and ensure your lab operates with precision and confidence.
Visual Guide
Related Products
- Ultra-Vacuum Electrode Feedthrough Connector Flange Power Electrode Lead for High-Precision Applications
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- 304 316 Stainless Steel Vacuum Ball Valve Stop Valve for High Vacuum Systems
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- KF ISO Stainless Steel Vacuum Flange Blind Plate for High Vacuum Systems
People Also Ask
- How do you maintain vacuum pressure? Master the balance between gas removal and gas load for stable performance.
- How is vacuum pressure measured? A Guide to Accurate Gauges and Techniques
- What are the units for vacuum pressure? Torr, mbar, and Pascal Explained
- What are the critical functions of Polytetrafluoroethylene (PTFE) gaskets within a glow discharge unit? Enhance Precision
- How thick is the thin film deposition? A Guide to Ranging from Nanometers to Micrometers



















