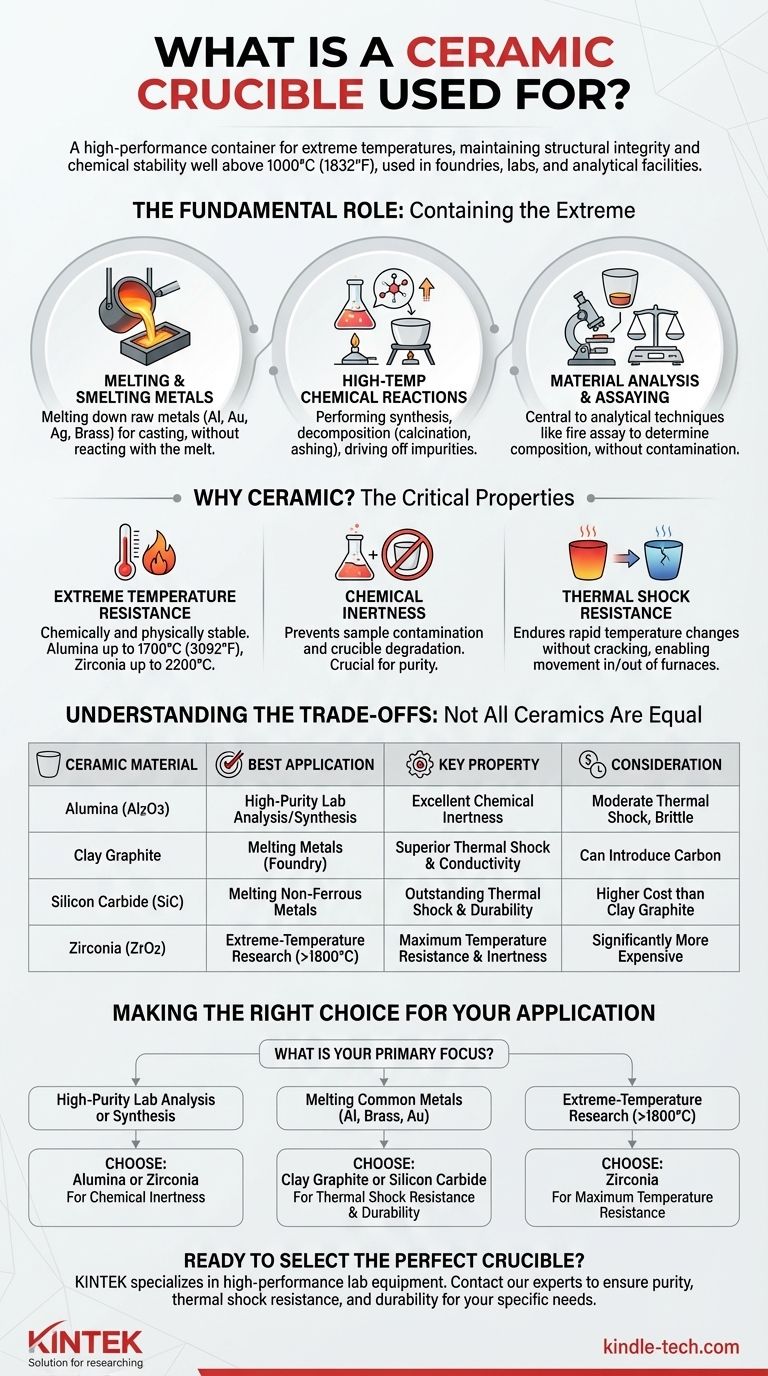
At its core, a ceramic crucible is a high-performance container designed to withstand extreme temperatures. It is used to hold materials for processes like melting metals, high-temperature chemical reactions, and material analysis. Unlike containers made of metal or glass, a ceramic crucible maintains its structural integrity and chemical stability well above 1000°C (1832°F), making it indispensable in foundries, laboratories, and analytical facilities.
The essential function of a ceramic crucible is to contain substances during high-temperature processes. However, the true value lies in selecting the right ceramic material—like alumina or silicon carbide—to ensure chemical inertness and thermal shock resistance for a specific application.

The Fundamental Role: Containing the Extreme
A crucible's primary job is to serve as a vessel in environments that would destroy ordinary containers. This function is critical across several scientific and industrial domains.
Melting and Smelting Metals
In foundries and for jewelers, crucibles are used to melt down raw metals like aluminum, gold, silver, and brass into a liquid state for casting. The crucible must contain the molten metal without reacting with it or failing under the intense heat of the furnace.
High-Temperature Chemical Reactions
Chemists and material scientists use crucibles to perform synthesis or decomposition of compounds at high temperatures. This includes processes like calcination, where a material is heated to drive off impurities, or ashing, where organic matter is burned away to measure the inorganic residue.
Material Analysis and Assaying
Crucibles are central to analytical techniques that determine a material's composition. In fire assay, for example, a crucible is used to separate precious metals like gold and silver from their ore by melting the sample with a series of fluxing agents. The crucible must not contaminate this sensitive process.
Why Ceramic? The Critical Properties
The choice of ceramic is not arbitrary. Specific material properties make ceramics uniquely suited for these high-heat tasks.
Extreme Temperature Resistance
This property, known as refractoriness, is the most important. Ceramics are materials that are chemically and physically stable at high temperatures. While steel melts around 1370°C (2500°F), an alumina ceramic crucible can be used at temperatures up to 1700°C (3092°F).
Chemical Inertness
The crucible material should not react with the substance it holds. This prevents contamination of the sample and degradation of the crucible itself. A pure metal alloy cannot be created in a crucible that leaches impurities into the melt.
Thermal Shock Resistance
Thermal shock is the stress a material endures when it experiences rapid temperature change, which can cause it to crack. Think of a hot glass dish shattering when placed in cold water. Crucibles with good thermal shock resistance can be moved in and out of a furnace without failing.
Understanding the Trade-offs: Not All Ceramics Are Equal
Choosing a crucible involves balancing performance with cost. The ideal material for one task may be unsuitable for another.
Alumina (Aluminum Oxide)
Alumina is a high-purity, widely used lab crucible. It offers excellent temperature resistance and chemical inertness, making it ideal for clean, analytical work. However, it can be brittle and has moderate thermal shock resistance.
Clay Graphite
Common in foundries, these crucibles are a mix of clay and graphite. The graphite provides excellent thermal conductivity, which allows for fast and even heating of the metal charge, and superior thermal shock resistance. The trade-off is that they can introduce carbon into the melt, which is undesirable for certain alloys.
Silicon Carbide (SiC)
Like clay graphite, SiC crucibles have outstanding thermal shock resistance and durability, making them a mainstay for melting non-ferrous metals like aluminum and brass. They are more durable and last longer than clay graphite crucibles but come at a higher cost.
Zirconia (Zirconium Dioxide)
Zirconia crucibles are a premium option used for applications requiring even higher temperatures than alumina can handle (up to 2200°C). They are exceptionally inert but are significantly more expensive, limiting their use to specialized research or industrial processes.
Making the Right Choice for Your Application
Your final selection depends entirely on the material you are working with and your goal.
- If your primary focus is high-purity lab analysis or synthesis: An alumina or zirconia crucible is your best choice for its chemical inertness.
- If your primary focus is melting common metals like aluminum, brass, or gold: A clay graphite or silicon carbide crucible offers the best balance of durability, thermal performance, and cost.
- If your primary focus is extreme-temperature research above 1800°C: Specialized materials like stabilized zirconia are required.
Understanding these properties transforms the crucible from a simple vessel into a precision instrument critical to your success.
Summary Table:
| Application | Recommended Crucible Type | Key Property |
|---|---|---|
| High-Purity Lab Analysis/Synthesis | Alumina or Zirconia | Chemical Inertness |
| Melting Metals (e.g., Aluminum, Gold) | Clay Graphite or Silicon Carbide | Thermal Shock Resistance & Durability |
| Extreme-Temperature Research (>1800°C) | Zirconia | Maximum Temperature Resistance |
Ready to select the perfect crucible for your high-temperature process? KINTEK specializes in high-performance lab equipment, including a full range of ceramic crucibles. Our experts can help you choose the right material—from alumina to zirconia—to ensure chemical purity, thermal shock resistance, and durability for your specific application. Contact our team today to discuss your needs and enhance your lab's capabilities!
Visual Guide
Related Products
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
People Also Ask
- How is a crucible used? A Guide to Safe and Efficient High-Temperature Melting
- Why are alumina or quartz crucibles used for Barium Titanate annealing? Ensure Purity in BTO Nanofiber Synthesis
- What material is used to make a crucible? The Ultimate Guide to Choosing the Right One
- What is the purpose of using an alumina crucible with mother powder for Ga-LLZO? Ensure Pure Sintering Results
- What are the advantages of a crucible furnace? Achieve Precision Melting for Labs & Small-Scale Production
- What is the role of a boat-shaped alumina crucible? Optimize LATP Solid-State Electrolyte Synthesis Efficiency
- What is the role of crucibles in the Pechini method? Key Steps for High-Purity Synthesis
- What is the role of grinding spent catalysts in a ceramic crucible? Unlock 99% Leaching Recovery Rates



















