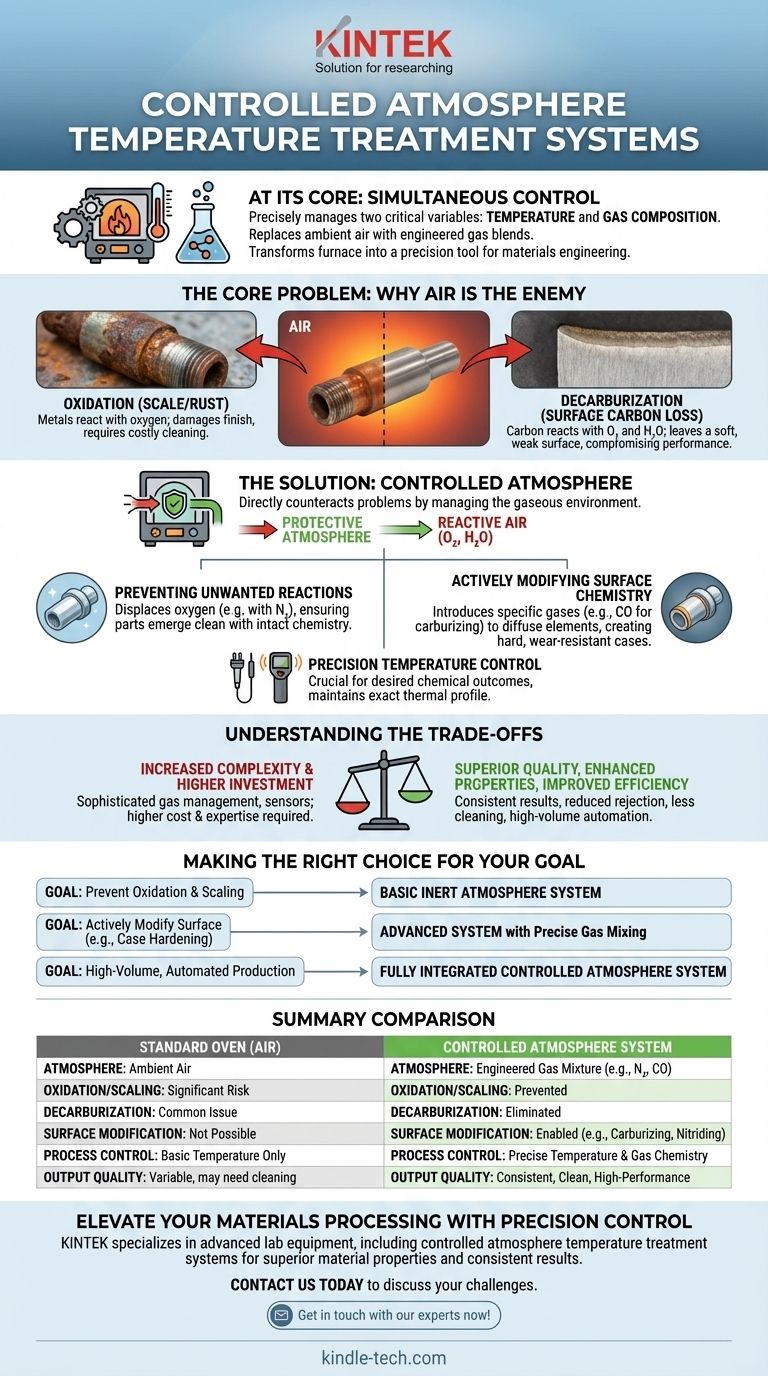
At its core, a Controlled Atmosphere Temperature Treatment System is an advanced industrial furnace or oven that precisely manages two critical variables simultaneously: temperature and the chemical composition of the gas surrounding the workpiece. Unlike a standard oven that heats in ambient air, this system replaces the air with a specific, engineered blend of gases. This control prevents unwanted chemical reactions and can be used to intentionally alter the surface properties of a material.
The fundamental purpose of this technology is to move beyond simple heating. It transforms a furnace from a blunt instrument into a precision tool for materials engineering, enabling you to protect a part's surface or fundamentally change its chemistry to achieve specific performance characteristics.

The Core Problem: Why Air Is the Enemy in Heat Treatment
To understand the value of a controlled atmosphere, you must first understand the problem it solves. Heating metals, particularly steel, in the presence of normal air creates significant issues.
The Threat of Oxidation
When heated, metals react with the oxygen in the air. This process, known as oxidation, forms a layer of scale or rust on the surface. This damages the part's finish, can alter its dimensions, and often requires costly and time-consuming secondary cleaning processes.
The Danger of Decarburization
For many steels, the carbon content at the surface is critical for its hardness and wear resistance. At high temperatures, the carbon in the steel can react with oxygen and water vapor in the air, effectively removing carbon from the surface layer. This phenomenon, called decarburization, leaves the part with a soft, weak surface, compromising its performance and fatigue life.
How a Controlled Atmosphere Provides the Solution
A controlled atmosphere system directly counteracts these problems by replacing the reactive air with a carefully managed gaseous environment.
Preventing Unwanted Reactions
The most basic function is protection. By introducing a protective atmosphere—often rich in nitrogen or other inert gases—the system displaces oxygen. This prevents oxidation and decarburization, ensuring the part emerges from the furnace clean and with its surface chemistry intact.
Actively Modifying Surface Chemistry
More advanced systems use the atmosphere to intentionally change the material. This is known as chemical heat treatment. By introducing specific "infiltrating" gases, you can add elements to the surface of the part. A common example is carburizing, where a carbon-rich atmosphere (using gases like CO) is used to diffuse carbon into the surface of low-carbon steel, creating a very hard, wear-resistant outer case.
The Need for Precision Temperature Control
The chemical reactions that occur during treatment are highly dependent on temperature. A controlled atmosphere is only effective when paired with a precise temperature management system. These systems use sensors like thermocouples (for lower ranges) or infrared instruments (for very high temperatures) to maintain the exact thermal profile required for the desired chemical outcome.
Understanding the Trade-offs
While powerful, this technology represents a significant step up from conventional heating methods, which comes with inherent trade-offs.
Increased System Complexity
These systems are not simple ovens. They require sophisticated gas mixing panels, flow controllers, seals to maintain the atmosphere, and sensors to monitor the gas composition. This adds layers of mechanical and electrical complexity.
Higher Initial Investment
The specialized equipment required for gas management and precise control makes controlled atmosphere furnaces more expensive than their conventional, air-based counterparts.
Greater Process Expertise Required
Operating these systems effectively demands a deeper understanding of material science and chemistry. Achieving consistent results requires knowledge of how to set gas ratios, flow rates, and temperature profiles for specific materials and desired outcomes.
Key Benefits of Adopting This Technology
The trade-offs are often justified by significant improvements in quality, efficiency, and capability.
Superior Product Quality and Consistency
By eliminating variables like air humidity and composition, these systems produce highly consistent and repeatable results. Parts emerge with clean surfaces and precisely controlled surface properties, drastically reducing rejection rates.
Enhanced Material Properties
The ability to prevent decarburization and perform chemical treatments like carburizing allows for the creation of parts with superior wear resistance and fatigue strength. This can enable the use of less expensive base materials that are then surface-hardened to meet demanding specifications.
Improved Overall Efficiency
While the equipment is complex, the process can be highly automated. It often eliminates the need for post-treatment cleaning, reduces material waste from scaling, and saves significant manpower, leading to a lower cost per part in high-volume production.
Making the Right Choice for Your Goal
The right system depends entirely on the specific outcome you need to achieve.
- If your primary focus is preventing simple oxidation and scaling: A system capable of providing a basic inert or non-oxidizing atmosphere is sufficient.
- If your primary focus is actively modifying surface properties (like case hardening): You need a more advanced system with precise gas mixing and monitoring capabilities for processes like carburizing or nitriding.
- If your primary focus is high-volume, automated production: Investing in a fully integrated controlled atmosphere system is justified by the major gains in consistency, reduced labor, and elimination of secondary processing steps.
Ultimately, adopting a controlled atmosphere system is about gaining precise control over the chemical destiny of your material during its most critical thermal processing stages.
Summary Table:
| Aspect | Standard Oven (Air) | Controlled Atmosphere System |
|---|---|---|
| Atmosphere | Ambient Air | Engineered Gas Mixture (e.g., N₂, CO) |
| Oxidation/Scaling | Yes, significant risk | Prevented |
| Decarburization | Yes, common issue | Eliminated |
| Surface Modification | Not possible | Enabled (e.g., Carburizing, Nitriding) |
| Process Control | Basic temperature only | Precise temperature & gas chemistry |
| Output Quality | Variable, may need cleaning | Consistent, clean, high-performance |
Ready to elevate your materials processing with precision control?
At KINTEK, we specialize in advanced lab equipment, including controlled atmosphere temperature treatment systems designed for laboratories and research facilities. Our solutions help you achieve superior material properties, prevent surface defects, and ensure consistent, high-quality results.
Contact us today to discuss your specific heat treatment challenges and discover how our expertise can enhance your laboratory's capabilities.
Get in touch with our experts now!
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- What key processing conditions does a tubular atmosphere furnace provide? Unlock Cr/SZ Catalyst Performance
- Are inert gases harmful to humans? The Silent Threat of Oxygen Displacement
- Is the atmosphere oxidizing or reducing? Discover the Chemical Engine of Our Planet
- What are the specific functions of a high-temperature carbonization furnace and an activation reactor in bamboo carbon?
- Why is it necessary to control atmosphere during sintering? Prevent Oxidation and Control Material Properties
- Why nitrogen is used in annealing process? Prevent Oxidation for a Perfect Metal Finish
- What are the disadvantages of an inert gas system? Understanding the Safety and Operational Risks
- Why is a high-precision atmosphere or vacuum sintering furnace essential for verifying Ti2AlC oxidation mechanisms?



















