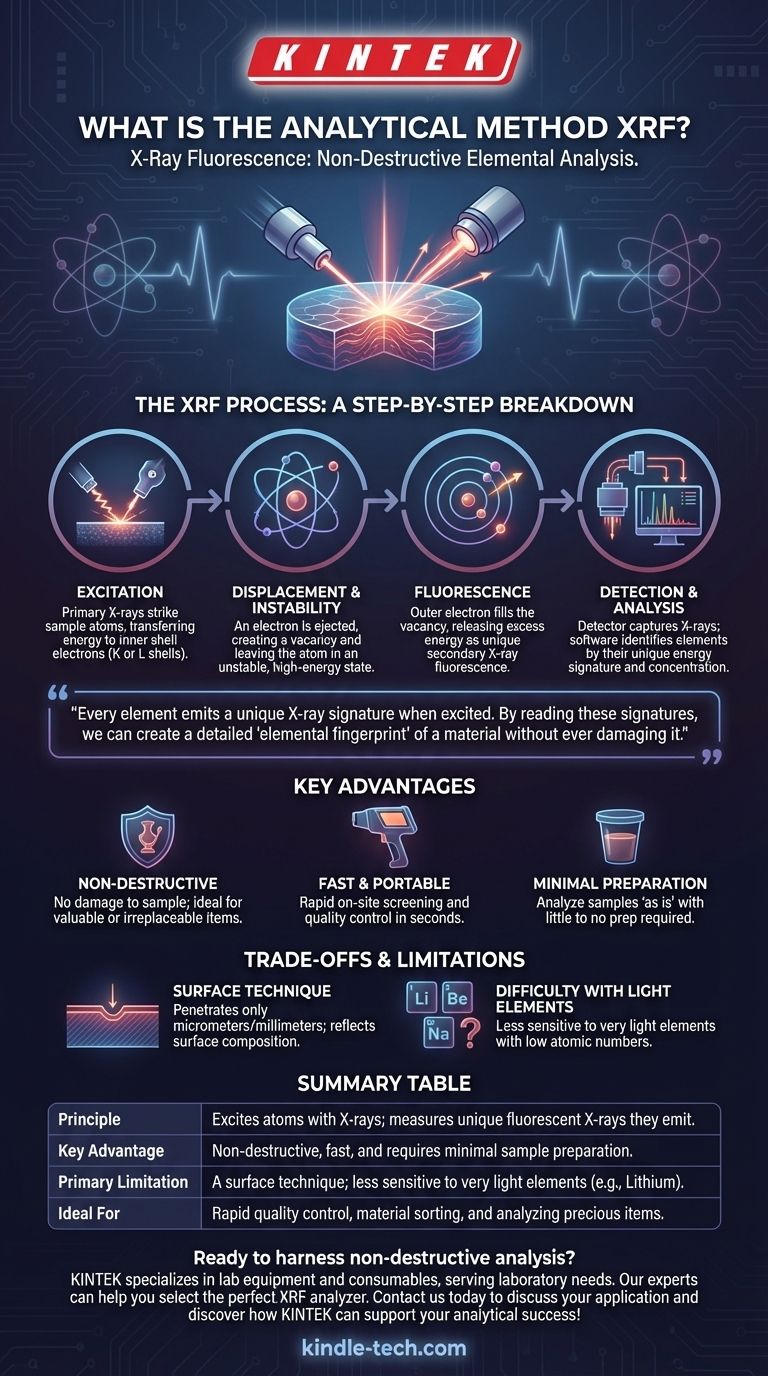
At its core, X-Ray Fluorescence (XRF) is a non-destructive analytical technique used to determine the elemental composition of materials. It works by bombarding a sample with high-energy X-rays, which causes the elements within that sample to emit their own characteristic "fluorescent" X-rays. By detecting and measuring these secondary X-rays, an XRF analyzer can identify which elements are present and in what quantities.
The central principle of XRF is that every element emits a unique X-ray signature when excited. By reading these signatures, we can create a detailed "elemental fingerprint" of a material without ever damaging it.

The XRF Process: A Step-by-Step Breakdown
To truly understand XRF, we need to look at the four distinct stages that occur in rapid succession within the analyzer. This entire process, from excitation to result, is often completed in just a few seconds.
Step 1: Excitation
The process begins when the XRF instrument fires a primary X-ray beam at the surface of the sample.
This incoming beam is composed of high-energy photons. When these photons strike atoms within the sample, they transfer their energy to electrons in the innermost electron shells (typically the K or L shells).
Step 2: Displacement and Instability
If the primary X-ray has sufficient energy, it will dislodge an electron from its inner shell, ejecting it from the atom entirely.
This event creates a vacancy, or "hole," in the electron shell, leaving the atom in an unstable, high-energy state. The atom immediately seeks to return to a more stable, lower-energy configuration.
Step 3: Fluorescence
To regain stability, an electron from a higher-energy outer shell drops down to fill the vacancy in the lower-energy inner shell.
As this electron moves from a high-energy state to a lower one, it releases the excess energy in the form of a secondary X-ray. This emission is known as X-ray fluorescence.
Step 4: Detection and Analysis
The energy of this emitted fluorescent X-ray is unique to the element from which it originated. It is a direct fingerprint of that specific atom.
An X-ray detector within the analyzer captures these secondary X-rays and counts them. The instrument's software then processes this data, identifying each element by its characteristic energy signature and determining its concentration based on the intensity of the signal.
Key Advantages of the XRF Method
The principles behind XRF give it several significant advantages that make it a preferred method in many industries, from geology and mining to manufacturing and recycling.
It Is Non-Destructive
Perhaps the most crucial benefit of XRF is that it does not damage the sample. The X-ray beam excites the atoms but does not alter the material's physical or chemical properties. This is essential for analyzing valuable or irreplaceable items, such as historical artifacts, jewelry, or critical manufacturing components.
It Is Fast and Portable
Modern XRF analyzers, particularly handheld models, can provide accurate elemental analysis in a matter of seconds. This allows for rapid on-site screening and quality control without the need to send samples to a laboratory, saving significant time and resources.
It Requires Minimal Sample Preparation
For many applications, samples can be analyzed "as is" with little to no preparation. This simplicity makes the workflow extremely efficient, especially when compared to other analytical techniques that require complex and time-consuming sample digestion or preparation protocols.
Understanding the Trade-offs and Limitations
While powerful, XRF is not without its limitations. Understanding these trade-offs is crucial for interpreting results correctly and knowing when to use the technique.
It Is Primarily a Surface Technique
The primary X-rays can only penetrate a very shallow depth into the sample (from micrometers to millimeters, depending on the material). Therefore, the results are only representative of the surface composition. If the material is coated, corroded, or not homogenous, the XRF reading may not reflect the bulk composition of the item.
It Has Difficulty with Light Elements
XRF is less sensitive to very light elements (those with low atomic numbers, like lithium, beryllium, or sodium). The fluorescent X-rays emitted by these elements are very low in energy and are often absorbed by the air or the detector window before they can be measured effectively.
Making the Right Choice for Your Goal
- If your primary focus is rapid quality control or material sorting: XRF is an ideal choice due to its speed, portability, and non-destructive nature, allowing for immediate pass/fail decisions on the factory floor or in the field.
- If your primary focus is analyzing precious or unique items: The non-destructive aspect of XRF is its greatest strength, providing detailed elemental data without causing any harm to the sample.
- If your primary focus is determining the bulk composition of a non-homogenous material: XRF may only be a starting point. You might need to use a different technique or prepare the sample (e.g., by grinding it into a powder) to get a truly representative analysis.
Ultimately, XRF provides a powerful and efficient window into the elemental world, enabling you to understand the fundamental building blocks of a material instantly.
Summary Table:
| Aspect | Description |
|---|---|
| Principle | Excites atoms with X-rays; measures unique fluorescent X-rays they emit. |
| Key Advantage | Non-destructive, fast, and requires minimal sample preparation. |
| Primary Limitation | A surface technique; less sensitive to very light elements (e.g., Lithium). |
| Ideal For | Rapid quality control, material sorting, and analyzing precious items. |
Ready to harness the power of non-destructive elemental analysis in your lab?
XRF technology can revolutionize your quality control and material verification processes, providing instant, accurate results without damaging your samples. Whether you're in mining, manufacturing, or research, having the right equipment is key.
KINTEK specializes in lab equipment and consumables, serving laboratory needs. Our experts can help you select the perfect XRF analyzer to meet your specific goals, ensuring you get precise, reliable data every time.
Contact us today to discuss your application and discover how KINTEK can support your analytical success!
Visual Guide
Related Products
- Laboratory Test Sieves and Sieving Machines
- Custom PTFE Teflon Parts Manufacturer for PTFE Tweezers
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Benchtop Laboratory Vacuum Freeze Dryer
People Also Ask
- What are ultralow temperature freezers and what are they used for? Preserve Critical Samples for Decades
- Is pyrolysis oil same as diesel? Uncover the Critical Differences in Fuel Properties
- What are the uses of furnace in chemistry laboratory? Unlock High-Temperature Material Synthesis and Analysis
- How hot can a metal surface get in the sun? The Surprising Science Behind Extreme Heat
- What are the challenges of biomass conversion? Overcoming Economic and Technical Hurdles
- What are the advantages of solid state sintering? Achieve High-Performance Parts Efficiently
- What is the importance of biomass pyrolysis? Turn Waste into Fuel, Carbon Sequestration, and More
- What are the methods of separation and purification? Master the Key Techniques for Your Lab



















