The best aluminum alloys for brazing are those with low magnesium content and a high solidus (melting) temperature. Specifically, alloys in the 1xxx, 3xxx, and 6xxx series are excellent candidates because their material properties are highly compatible with the brazing process. Alloys like 3003 and 6061 are commonly used for their good balance of formability, strength, and superior brazability.
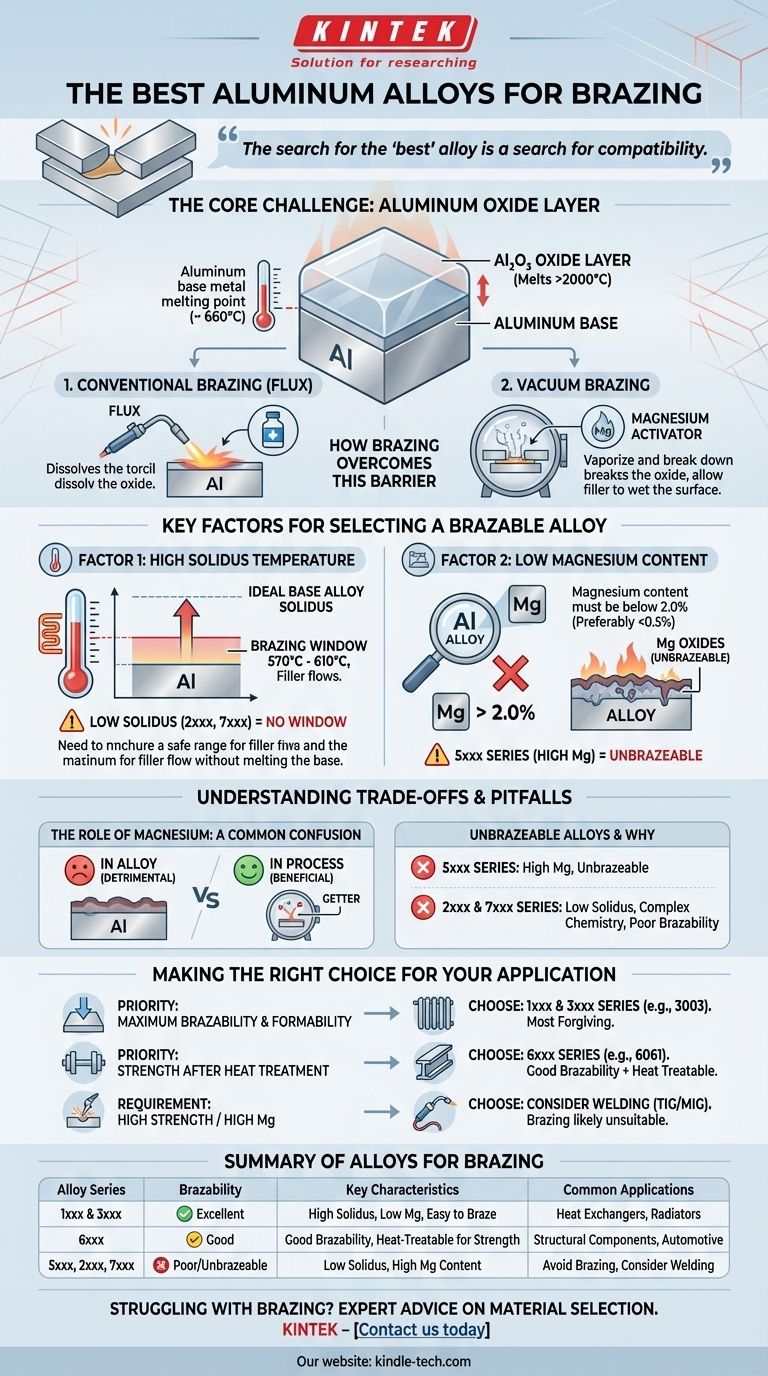
The search for the "best" alloy is fundamentally a search for compatibility. Successful aluminum brazing depends less on finding a single perfect material and more on understanding the two critical constraints of the process: the alloy's melting point must be safely above the filler metal's flow point, and its magnesium content must be low enough to prevent the formation of a stubborn, non-brazeable oxide layer.

The Core Challenge: The Aluminum Oxide Layer
Why Aluminum is Difficult to Join
All aluminum alloys are instantly covered by a thin, tough, and transparent layer of aluminum oxide (Al₂O₃).
This oxide layer has a melting point of over 2000°C (3632°F), which is far higher than the melting point of the aluminum base metal itself (around 660°C or 1220°F).
For a brazing filler metal to bond with the aluminum, this tenacious oxide layer must first be removed or displaced.
How Brazing Overcomes This Barrier
Brazing processes use one of two methods to defeat the oxide layer. In conventional furnace or torch brazing, a chemical flux is used to dissolve and displace the oxide.
In vacuum brazing, the parts are heated in a high vacuum. This environment, combined with a metallic activator like magnesium vapor, causes the oxide layer to break down, allowing the filler metal to wet the clean aluminum surface beneath.
Key Factors for Selecting a Brazable Alloy
Factor 1: High Solidus Temperature
The solidus is the temperature at which an alloy begins to melt. For brazing to work, the solidus temperature of the base metal must be significantly higher than the liquidus (full flow) temperature of the brazing filler metal.
This creates a "brazing window"—a safe temperature range where the filler is fully molten but the base material remains solid and stable.
Most aluminum brazing fillers flow between 570°C and 610°C (1060°F and 1130°F). Therefore, an ideal base alloy should not begin to melt until well above this range.
Factor 2: Low Magnesium Content
Magnesium is the single most disruptive element for aluminum brazing. As a rule, magnesium content in the base alloy must be below 2.0%, with many experts preferring to stay below 0.5% for best results.
When an aluminum alloy containing magnesium is heated, it forms magnesium oxides on the surface. These oxides are far more stable and difficult for chemical fluxes or vacuum processes to remove than pure aluminum oxide.
This is why the entire 5xxx series of alloys (which are strengthened with magnesium) are generally considered unbrazeable.
Understanding the Trade-offs and Pitfalls
The Role of Magnesium: A Common Point of Confusion
It is critical to distinguish between magnesium in the alloy and magnesium used in the process.
Magnesium as an alloying element (e.g., in the 5xxx series) is detrimental because it creates a refractory oxide layer on the part itself.
Conversely, small amounts of pure magnesium are often intentionally placed inside a vacuum furnace. Here, it acts as a "getter," vaporizing and reacting with any residual oxygen or water vapor in the vacuum, which helps protect the aluminum parts and break down their oxide layers.
Unbrazeable and Difficult-to-Braze Alloys
Based on the principles above, some alloy series should be avoided for brazing applications.
- 5xxx Series: These alloys are strengthened with high levels of magnesium and are considered unbrazeable.
- 2xxx and 7xxx Series: These high-strength alloys often have low solidus temperatures that leave little to no brazing window. Their complex chemistries also interfere with the process.
Making the Right Choice for Your Application
The ideal alloy depends on whether your priority is ease of brazing, post-braze strength, or other manufacturing considerations.
- If your primary focus is maximum brazability and formability: Choose alloys from the 1xxx (pure aluminum) or 3xxx (aluminum-manganese) series, as they are the most forgiving.
- If your primary focus is strength after heat treatment: Use an alloy from the 6xxx series, such as 6061 or 6063, which offers a great balance of good brazability and the ability to be heat-treated to a higher strength after joining.
- If your project requires a high-magnesium or high-strength alloy (5xxx, 2xxx, 7xxx): Recognize that brazing is likely the wrong joining method and investigate alternatives like TIG or MIG welding.
Choosing the right material from the outset, based on these principles, is the most critical step toward a successful brazed aluminum joint.
Summary Table:
| Alloy Series | Brazability | Key Characteristics | Common Applications |
|---|---|---|---|
| 1xxx & 3xxx | Excellent | High solidus temp, low Mg, easy to braze | Heat exchangers, radiators |
| 6xxx | Good | Good brazability, can be heat-treated for strength | Structural components, automotive parts |
| 5xxx, 2xxx, 7xxx | Poor/Unbrazeable | Low solidus temp, high Mg content | Avoid for brazing; consider welding instead |
Struggling with aluminum brazing or need expert advice on material selection for your lab or production needs?
At KINTEK, we specialize in providing high-quality lab equipment and consumables tailored to your specific requirements. Whether you're working on brazing projects or other laboratory processes, our expertise ensures you get the right tools for optimal results.
Contact us today to discuss how we can support your laboratory's success with reliable equipment and expert guidance!
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- Can dissimilar metals be brazed or braze welded? A Guide to Strong, Reliable Joints
- What is brazing in heat treatment? Achieve Superior Joint Quality and Efficiency
- Where are vacuum furnaces used? Essential for High-Purity Heat Treatment in Critical Industries
- What is the cost of a vacuum brazing furnace? A guide to key factors and investment strategy
- What is vacuum brazing? The Ultimate Guide to High-Purity, Flux-Free Metal Joining



















