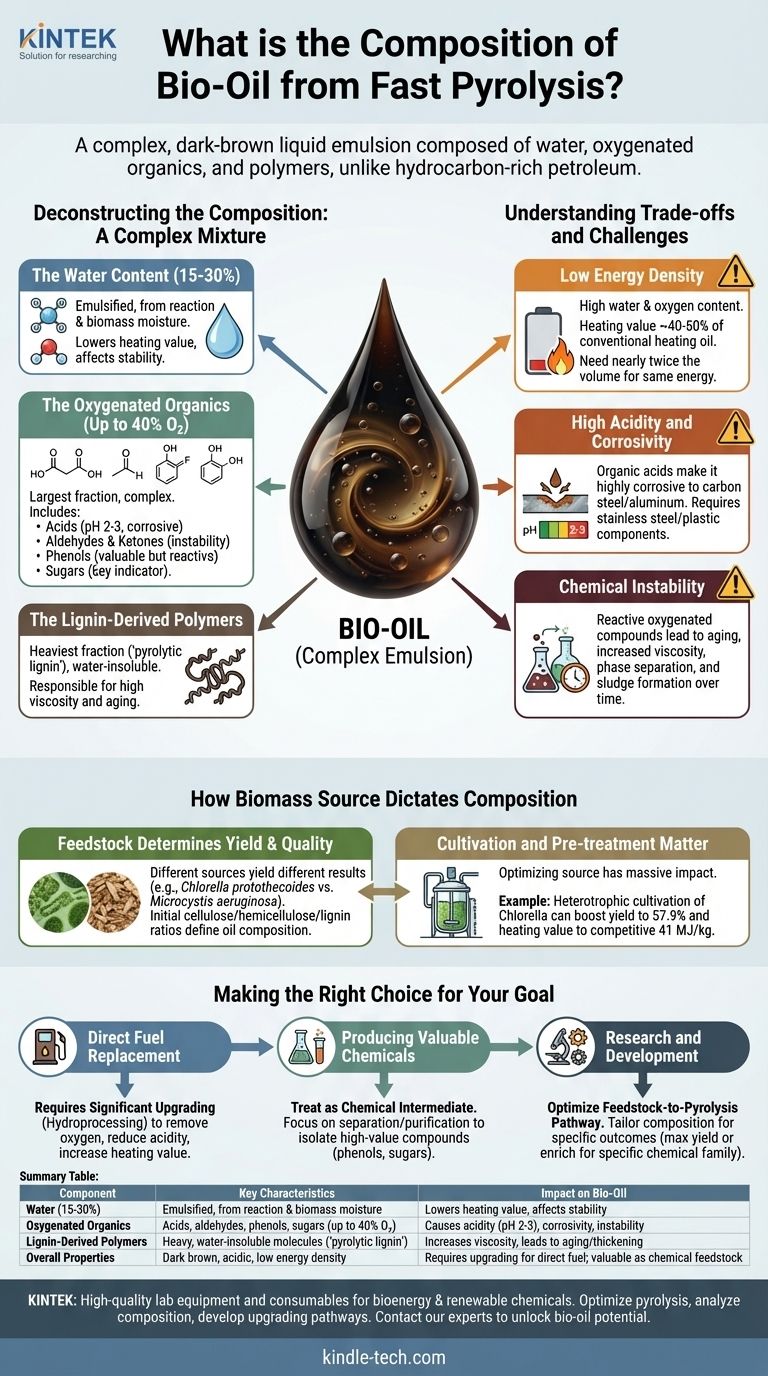
At its core, bio-oil from fast pyrolysis is a complex, dark-brown liquid emulsion composed primarily of water, highly oxygenated organic compounds, and polymers. Unlike petroleum crude oil, which is a mix of hydrocarbons, bio-oil's composition includes hundreds of different chemicals, with oxygen content as high as 40% by weight. This mixture ranges from simple compounds like acetic acid and formaldehyde to larger, more complex phenols and sugar-derived molecules.
Bio-oil's chemical makeup is its greatest challenge and its most significant opportunity. The high concentration of water and reactive oxygenated compounds makes it acidic, unstable, and lower in energy content than fossil fuels, but also positions it as a potential feedstock for renewable chemicals and upgraded biofuels.

Deconstructing the Composition: A Complex Mixture
Understanding bio-oil requires breaking it down into its primary chemical families. The exact proportions vary significantly based on the biomass feedstock and pyrolysis conditions, but the fundamental components remain consistent.
The Water Content
A significant fraction of bio-oil is water, formed during the pyrolysis reaction and originating from the moisture in the initial biomass. This water is finely emulsified within the oil phase, not separate from it.
The presence of water directly contributes to the oil's lower heating value compared to petroleum fuels and can affect its long-term stability.
The Oxygenated Organics
This is the largest and most complex fraction of bio-oil, making it fundamentally different from hydrocarbons. These compounds are responsible for most of the oil's characteristic properties.
Key groups include:
- Acids: Primarily acetic and formic acid, which make the bio-oil highly acidic (pH 2-3) and corrosive.
- Aldehydes & Ketones: Simple, reactive compounds like formaldehyde and hydroxyacetone contribute to the oil's instability.
- Phenols: Derived from the lignin in biomass, these compounds are valuable chemical precursors but also contribute to the oil's reactivity.
- Sugars: Anhydrosugars like levoglucosan are formed from the breakdown of cellulose and are a key indicator of pyrolysis efficiency.
The Lignin-Derived Polymers
The heaviest fraction of bio-oil consists of large, water-insoluble molecules often called "pyrolytic lignin." These are partially broken-down polymers from the original biomass.
These polymers are responsible for the high viscosity of bio-oil and its tendency to thicken or even solidify over time through further polymerization reactions.
Understanding the Trade-offs and Challenges
The unique composition of raw bio-oil creates several significant hurdles for its direct use as a "drop-in" fuel, making upgrading a near necessity.
Low Energy Density
Due to its high water and oxygen content, the heating value of bio-oil is roughly 40-50% that of conventional heating oil. This means you need nearly twice the volume of bio-oil to produce the same amount of energy.
High Acidity and Corrosivity
The presence of organic acids makes raw bio-oil highly corrosive to common construction metals like carbon steel and aluminum. This necessitates the use of more expensive stainless steel or plastic for storage tanks, pipes, and engine components.
Chemical Instability
The wide array of reactive oxygenated compounds (aldehydes, phenols) in bio-oil means it is not chemically stable. Over time, these molecules react with each other, increasing the oil's viscosity, causing phase separation, and forming sludge. This aging process complicates long-term storage and transportation.
How Biomass Source Dictates Composition
The composition of bio-oil is not fixed; it is a direct reflection of the biomass it came from. The type of feedstock and even its cultivation method can dramatically alter the final product.
Feedstock Determines Yield and Quality
Different biomass sources produce different results. For example, fast pyrolysis of the algae Chlorella protothecoides yields about 18% bio-oil, while Microcystis aeruginosa yields 24%. The initial ratios of cellulose, hemicellulose, and lignin in the feedstock will define the resulting ratio of sugars, acids, and phenols in the oil.
Cultivation and Pre-treatment Matter
Optimizing the biomass source can have a massive impact. For instance, a standard culture of Chlorella protothecoides might yield 18% bio-oil. However, using a heterotrophic cultivation method can boost that yield to 57.9% while also increasing the heating value to 41 MJ/kg, which is competitive with fossil fuels.
Making the Right Choice for Your Goal
Understanding bio-oil's composition is the first step to leveraging it effectively for a specific application. Your strategy will depend entirely on your end goal.
- If your primary focus is direct fuel replacement: You must plan for significant upgrading, such as hydroprocessing, to remove oxygen, reduce acidity, and increase the heating value.
- If your primary focus is producing valuable chemicals: Treat the bio-oil as a chemical intermediate, focusing on separation and purification techniques to isolate high-value compounds like phenols or specific sugars.
- If your primary focus is research and development: Concentrate on optimizing the feedstock-to-pyrolysis pathway to tailor the bio-oil's composition for a desired outcome, whether it's maximizing yield or enriching for a specific chemical family.
Ultimately, viewing bio-oil not as a flawed version of crude oil, but as a unique chemical intermediate, unlocks its true potential in a renewable future.
Summary Table:
| Component | Key Characteristics | Impact on Bio-Oil |
|---|---|---|
| Water (15-30%) | Emulsified, from reaction & biomass moisture | Lowers heating value, affects stability |
| Oxygenated Organics | Acids, aldehydes, phenols, sugars (up to 40% O₂) | Causes acidity (pH 2-3), corrosivity, and instability |
| Lignin-Derived Polymers | Heavy, water-insoluble molecules ('pyrolytic lignin') | Increases viscosity, leads to aging/thickening |
| Overall Properties | Dark brown, acidic, low energy density (~40-50% of fossil fuel) | Requires upgrading for direct fuel use; valuable as chemical feedstock |
Ready to transform your biomass research or production process?
Understanding the complex composition of bio-oil is just the first step. Successfully converting biomass into valuable products requires precise control and reliable equipment for pyrolysis, analysis, and upgrading.
At KINTEK, we specialize in providing high-quality lab equipment and consumables tailored to the needs of researchers and developers in the bioenergy and renewable chemicals sector. Whether you are optimizing pyrolysis conditions, analyzing bio-oil composition, or developing upgrading pathways, our solutions can help you achieve accurate, reproducible results.
Let us help you unlock the potential of bio-oil. Contact our experts today to discuss how our equipment can support your specific goals, from maximizing yield to isolating high-value chemicals.
Visual Guide
Related Products
- Square Lab Press Mold for Laboratory Applications
- Special Shape Press Mold for Lab
- Customizable CO2 Reduction Flow Cell for NRR ORR and CO2RR Research
- Customizable Fuel Cell Stack Components for Diverse Applications
- Thin-Layer Spectral Electrolysis Electrochemical Cell
People Also Ask
- What are the advantages of using PEEK molds for sulfide all-solid-state batteries? High Performance and Insulation
- What are the precautions to be taken while sampling? Ensure Data Accuracy and Minimize Bias
- What technical characteristics are required for specialty pressure molds used in the compaction of Li10GeP2S12? Expert Tips
- How do custom graphite molds contribute to Al-20% Si/graphite flake composites? Optimize Microstructure & Conductivity
- What are the factors affecting sample size requirements? Master the Trade-Offs for Credible Research



















