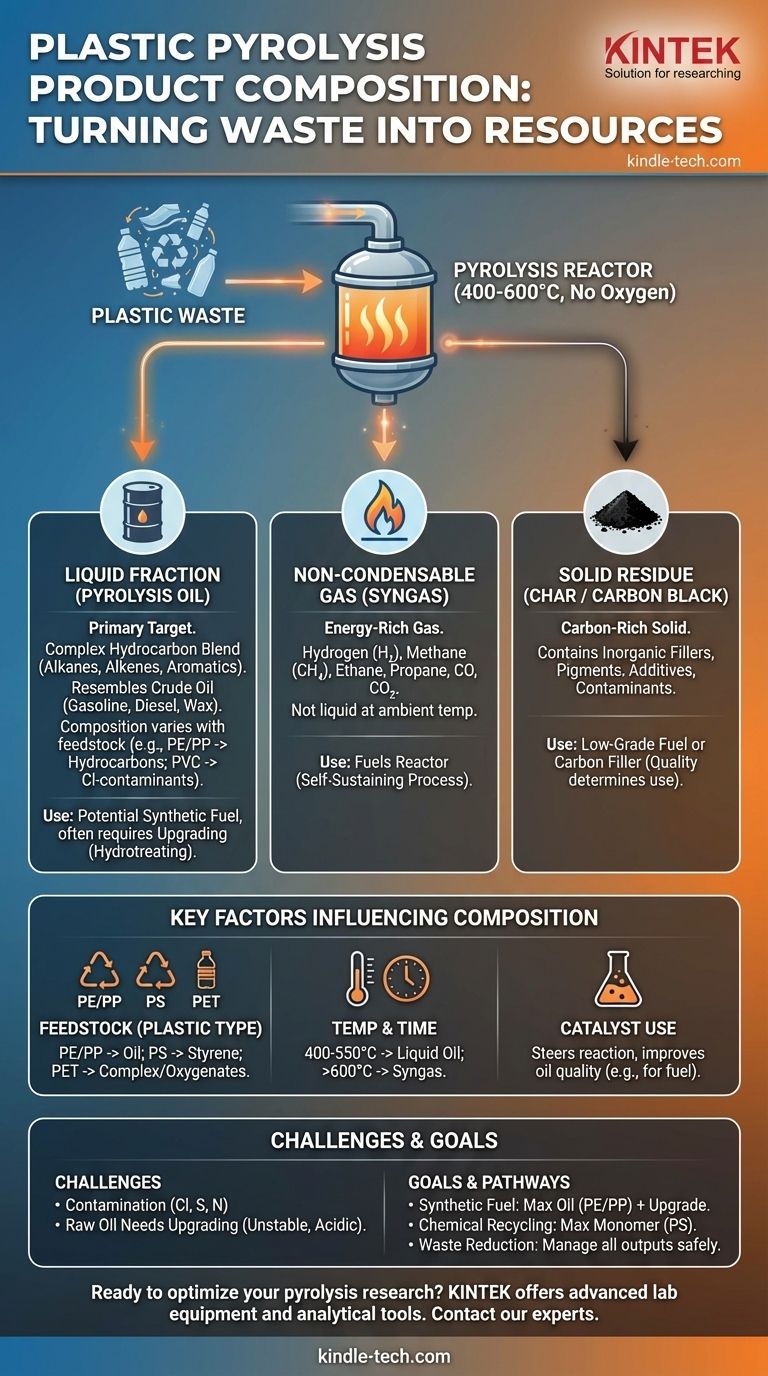
In short, plastic pyrolysis breaks down waste plastics into three primary products: a liquid hydrocarbon mixture called pyrolysis oil, a mix of non-condensable gases (syngas), and a solid residue (char). The exact composition of these products is not fixed; it varies significantly based on the type of plastic being processed and the specific conditions of the pyrolysis reaction.
The core challenge—and opportunity—of plastic pyrolysis is that it doesn't produce a single, clean output. Instead, it creates a complex, variable stream of liquid, gas, and solid materials that require careful management and often further processing to become valuable.

The Three Core Products of Plastic Pyrolysis
Pyrolysis is thermal decomposition in the absence of oxygen. When applied to plastics, it breaks long polymer chains into smaller, more useful molecules. These molecules separate into liquid, gas, and solid fractions.
Pyrolysis Oil (The Liquid Fraction)
This liquid is the primary target for most pyrolysis operations and is often considered a type of synthetic crude oil.
Its composition is a complex blend of hydrocarbon molecules. Unlike the referenced description of biomass pyrolysis oil, which is high in oxygen (up to 40%), oil from common plastics like polyethylene (PE) and polypropylene (PP) is primarily hydrocarbons with very little oxygen.
The liquid contains a wide spectrum of organic compounds, from lighter gasoline-range molecules to heavier diesel and wax fractions. The presence of specific plastics like PET can introduce oxygenated compounds, while PVC can introduce chlorine, making the oil corrosive and environmentally hazardous without further treatment.
Non-Condensable Gas (Syngas)
This is the portion of the output that does not turn into a liquid when cooled after leaving the reactor.
This gas is rich in energy and typically includes hydrogen, methane, ethane, propane, and butane. It also contains carbon monoxide (CO) and carbon dioxide (CO2).
In most commercial plants, this syngas is captured and used as a fuel to heat the pyrolysis reactor, making the process more energy-efficient and self-sustaining.
Solid Residue (Char or Carbon Black)
After the volatile components have been driven off, a solid, carbon-rich material is left behind.
This residue is primarily carbon, but it is not pure. It acts as a sink for inorganic materials present in the original plastic waste, such as fillers, pigments, additives (like glass fibers), and other contaminants.
The quality and purity of the char determine its use. Low-quality char may be used as a low-grade solid fuel, while higher-purity carbon black can potentially be sold as a filler for rubber or asphalt.
Key Factors Influencing Product Composition
You cannot understand the output without understanding the inputs and the process. The product mix is not static; it is a direct result of key operational variables.
The Type of Plastic Feedstock
This is the single most important factor. Different polymers break down into different products.
- Polyolefins (PE, PP): Produce a paraffin- and olefin-rich oil resembling crude oil, with a mix of gasoline, diesel, and wax fractions.
- Polystyrene (PS): Primarily breaks down into styrene monomer, making it an ideal candidate for true chemical recycling back into new polystyrene.
- PET (Polyethylene Terephthalate): Yields a more complex output, including oxygenated compounds and solid terephthalic acid, which complicates its use as a fuel.
Pyrolysis Temperature and Reaction Time
The conditions inside the reactor dictate what is produced.
- Lower Temperatures (approx. 400-550°C): This range typically maximizes the yield of liquid pyrolysis oil.
- Higher Temperatures (>600°C): Higher heat and longer reaction times tend to "crack" the molecules further, favoring the production of non-condensable gas over liquid oil.
The Role of Catalysts
Introducing a catalyst into the process can steer the chemical reactions toward a more specific and valuable output.
Catalysts can improve the quality of the pyrolysis oil by narrowing the range of hydrocarbon molecules produced, often favoring valuable gasoline-range aromatics. This can create a higher-quality drop-in fuel but adds complexity and cost to the operation.
Understanding the Trade-offs and Challenges
Objectivity requires acknowledging that pyrolysis products are not a perfect solution. They come with significant challenges that must be managed.
Contamination is Unavoidable
Unless the plastic feedstock is perfectly clean and sorted, contaminants will end up in the products.
Chlorine from PVC is a major issue, as it forms hydrochloric acid, which is highly corrosive and requires removal. Sulfur and nitrogen from certain plastics can also end up in the oil, requiring hydrotreating similar to conventional crude oil refining.
Raw Pyrolysis Oil Requires Upgrading
The raw liquid product is rarely a "drop-in" replacement for conventional fuels or chemical feedstocks.
It is often unstable, acidic, and contains a mix of undesirable compounds. To be used in refineries or as a finished fuel, it almost always requires a secondary upgrading process, such as hydrotreating, to remove contaminants and saturate unstable olefinic compounds.
Making the Right Choice for Your Goal
The "best" product composition depends entirely on your objective. Pyrolysis is a tool, and its output must be matched to a specific end-use.
- If your primary focus is creating synthetic fuel: Maximize the liquid oil yield from polyolefin feedstocks (PE, PP) and plan for the necessary capital and operational expense of an oil upgrading unit.
- If your primary focus is circular chemical recycling: Use a clean, single-stream feedstock like polystyrene to maximize the recovery of valuable styrene monomer for new plastic production.
- If your primary focus is waste volume reduction: Recognize that all three products (oil, gas, char) must have a defined and environmentally sound disposal or utilization pathway.
Ultimately, harnessing the potential of plastic pyrolysis depends on a clear understanding of the complex and variable nature of its products.
Summary Table:
| Product | Primary Composition | Key Characteristics |
|---|---|---|
| Pyrolysis Oil | Hydrocarbons (Alkanes, Alkenes, Aromatics) | Viscous liquid, resembles crude oil; quality depends on feedstock. |
| Non-Condensable Gases (Syngas) | Hydrogen (H₂), Methane (CH₄), Ethane, Propane, CO, CO₂ | Used to fuel the pyrolysis reactor for energy efficiency. |
| Solid Residue (Char) | Carbon, Inorganic Additives, Contaminants | Quality varies; can be used as fuel or filler if pure enough. |
| Key Influencing Factors | Impact on Composition | |
| Feedstock (Plastic Type) | Polyolefins (PE, PP) yield oil; Polystyrene yields styrene; PET yields oxygenates. | |
| Temperature & Time | Lower temps (400-550°C) favor oil; higher temps (>600°C) favor gas. | |
| Use of Catalyst | Can narrow hydrocarbon range, improving oil quality for fuel applications. |
Ready to turn plastic waste into valuable resources? KINTEK specializes in advanced laboratory equipment for pyrolysis research and process development. Whether you are analyzing feedstock, optimizing reaction conditions, or characterizing pyrolysis oil, gas, and char products, our precision ovens, reactors, and analytical tools provide the reliability and control you need. Let us help you achieve your recycling and energy recovery goals. Contact our experts today to find the right solution for your lab.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
People Also Ask
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success



















