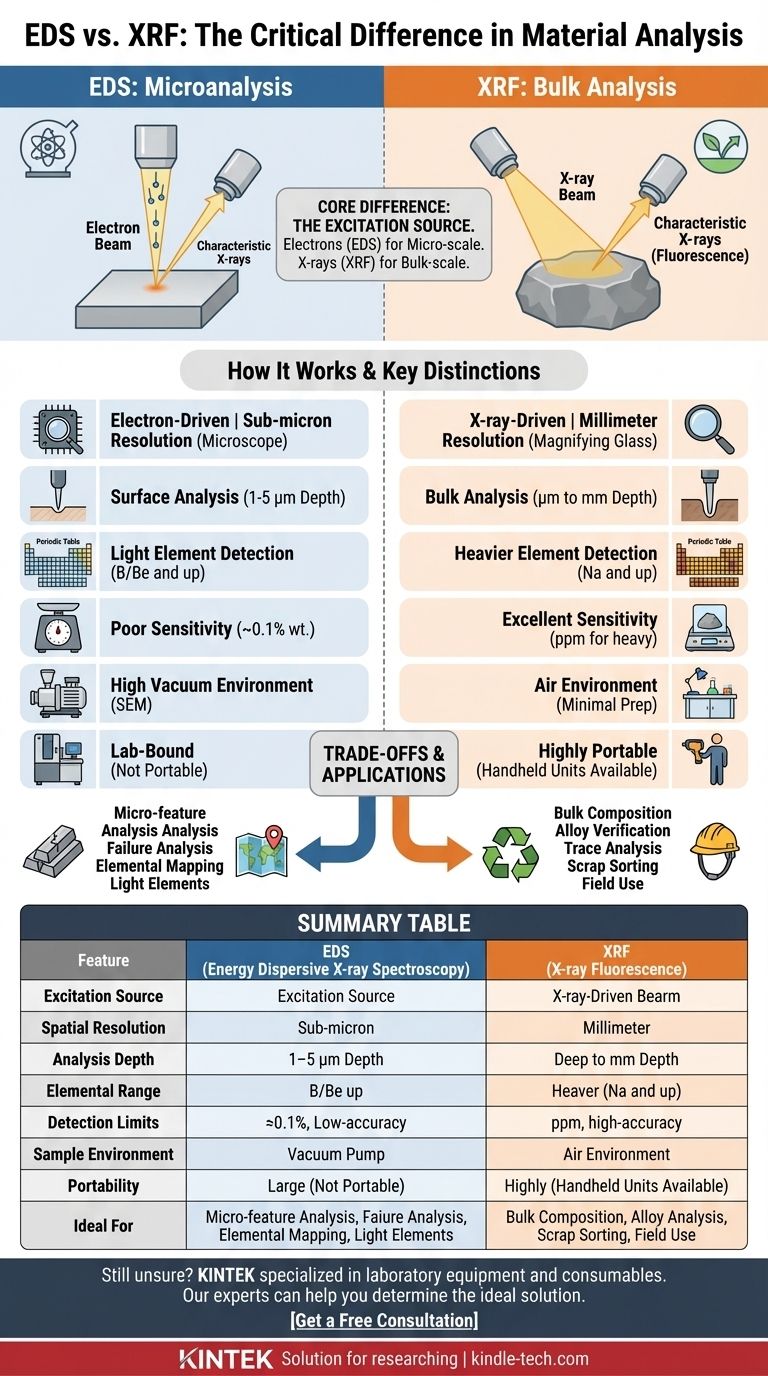
At its core, the difference is the excitation source. Energy Dispersive X-ray Spectroscopy (EDS) uses a focused beam of electrons to analyze a microscopic area, making it a tool for microanalysis. In contrast, X-ray Fluorescence (XRF) uses a beam of X-rays to analyze a much larger area, making it a tool for bulk chemical analysis. This single difference dictates their respective strengths, applications, and limitations.
The choice between EDS and XRF boils down to a simple question of scale. If you need to know the elemental composition of a microscopic feature, you need EDS. If you need to know the average elemental composition of a larger object or material, you need XRF.
How Each Technique Works: The Critical Distinction
Both EDS and XRF are powerful techniques for determining the elemental makeup of a material. They operate on the same fundamental principle: an incoming energy particle strikes an atom, ejects an inner-shell electron, and causes the atom to emit a "characteristic" X-ray whose energy level acts as a fingerprint for that specific element. The key is what type of particle starts this process.
The EDS Process: An Electron-Driven Technique
EDS (also called EDX) is not a standalone instrument; it is an accessory attached to a Scanning Electron Microscope (SEM).
An SEM uses a highly focused beam of electrons to scan a sample's surface. When these electrons hit the sample, they generate the characteristic X-rays that the EDS detector collects and analyzes.
Because the electron beam can be focused to a spot less than a nanometer wide, EDS can provide elemental information from an incredibly small volume. This makes it the ideal tool for analyzing tiny particles, thin films, or specific features on a complex surface.
The XRF Process: An X-ray-Driven Technique
An XRF instrument uses a small X-ray tube to generate a primary beam of high-energy X-rays.
This X-ray beam is directed at the sample. When the primary X-rays strike the atoms, they generate the secondary, characteristic X-rays (this is the "fluorescence" effect) that are then measured by a detector.
The X-ray beam in an XRF is orders of magnitude wider than an SEM's electron beam, typically ranging from millimeters to centimeters. This means XRF measures the average composition over a much larger and deeper area.
Key Performance Differences: A Practical Comparison
The difference in excitation source creates a cascade of practical differences in performance, defining which tool is right for a given job.
Spatial Resolution: Microscope vs. Magnifying Glass
EDS provides exceptional spatial resolution, often in the sub-micron range. It can create detailed "elemental maps" showing exactly where different elements are distributed across a microscopic surface.
XRF is a bulk analysis technique with low spatial resolution. It provides a single, averaged composition for the entire area illuminated by its wide X-ray beam.
Analysis Depth: Surface vs. Bulk
EDS is a surface-sensitive technique. The electrons only penetrate about 1-5 micrometers into the sample, meaning it only analyzes the very top layer of the material.
XRF analyzes a much deeper volume. The more energetic primary X-rays can penetrate from several micrometers to several millimeters, depending on the material's density. This gives a more representative analysis of the bulk material.
Elemental Range and Sensitivity
EDS can detect very light elements, often down to Boron (B) or even Beryllium (Be) with modern detectors. However, its detection limits are relatively poor, typically around 0.1% by weight, making it unsuitable for trace element analysis.
XRF generally cannot detect elements lighter than Sodium (Na). Its major strength is its excellent sensitivity for heavier elements, with detection limits often in the parts-per-million (ppm) range, making it the superior choice for trace analysis.
Understanding the Trade-offs
Choosing between these techniques also involves considering practical factors like sample preparation, cost, and portability.
Sample Preparation and Environment
EDS analysis takes place inside the high-vacuum chamber of an SEM. Samples must be small enough to fit, stable in a vacuum, and electrically conductive. Non-conductive samples (like plastics or ceramics) must be coated with a thin layer of carbon or gold.
XRF requires little to no sample preparation. It operates in air and can analyze solids, powders, or liquids. This flexibility is a significant advantage for rapid screening.
Portability and Field Use
EDS is exclusively a laboratory instrument, tethered to a large, immobile SEM.
XRF instruments are available as highly portable, handheld units. This allows for immediate, on-site analysis in applications like scrap metal sorting, mining exploration, and environmental testing.
Destructive vs. Non-Destructive
Both techniques are considered non-destructive, as they do not consume the sample.
However, the intense electron beam in EDS can sometimes cause localized damage or changes to sensitive materials like polymers, organic tissues, or glasses. XRF is generally less invasive.
Making the Right Choice for Your Goal
Your application's specific goal will be the ultimate guide.
- If your primary focus is failure analysis of a microscopic contaminant: You need the high spatial resolution of EDS to isolate and identify the tiny feature.
- If your primary focus is verifying the alloy grade of a large metal component: You need the bulk analysis and portability of a handheld XRF.
- If your primary focus is detecting trace amounts of lead in paint or toys: You need the superior sensitivity of XRF for heavy elements.
- If your primary focus is mapping the distribution of carbon across a polished cross-section: You need the light-element detection capability of EDS.
- If your primary focus is rapid quality control of raw materials on a factory floor: You need the speed and minimal sample prep of XRF.
Understanding this fundamental difference between an electron probe and an X-ray probe is the key to selecting the right tool for your analytical challenge.
Summary Table:
| Feature | EDS (Energy Dispersive X-ray Spectroscopy) | XRF (X-ray Fluorescence) |
|---|---|---|
| Excitation Source | Focused electron beam | X-ray beam |
| Spatial Resolution | Sub-micron (microscopic) | Millimeters to centimeters (bulk) |
| Analysis Depth | Surface (1-5 µm) | Deep (µm to mm) |
| Elemental Range | Light elements (Boron/Beryllium and up) | Heavier elements (Sodium and up) |
| Detection Limits | ~0.1% (weight) | Parts-per-million (ppm) for heavy elements |
| Sample Environment | High vacuum (SEM) | Air (minimal prep) |
| Portability | Lab-bound | Handheld units available |
| Ideal For | Micro-features, particles, thin films | Bulk composition, trace analysis, field use |
Still unsure which technique is right for your specific application?
KINTEK specializes in laboratory equipment and consumables, serving diverse analytical needs. Our experts can help you determine whether EDS or XRF is the ideal solution for your project, ensuring you get accurate and reliable results.
Contact us today to discuss your analytical challenges and discover how our solutions can enhance your lab's capabilities.
Visual Guide
Related Products
- XRD Sample Holder X-ray Diffractometer Powder Slide
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- XRF & KBR plastic ring lab Powder Pellet Pressing Mold for FTIR
- XRF Boric Acid Lab Powder Pellet Pressing Mold for Laboratory Use
People Also Ask
- What creates heat in a hydraulic system? Understanding Energy Loss and Pressure Drop
- Why is powder metallurgy limited to small parts? The Compaction & Cost Challenges Explained
- What role does a laboratory hydraulic press play in the preparation of solid electrolyte pellets? Ensure Data Accuracy
- How are laboratory pellet presses or rolling machines utilized in the preparation of LCO-LSLBO composite cathode sheets?
- What is a growth cell and what are its essential components in the HPHT process? Master Diamond Synthesis Core
- What types of hydraulic press systems are used for research? Discover Pellet, Hot, and Isostatic Solutions
- What is the advantage of a filter press? Achieve Maximum Dewatering & Slash Disposal Costs
- What steel is used for a hydraulic press? Choosing the Right Materials for High-Stress Performance







